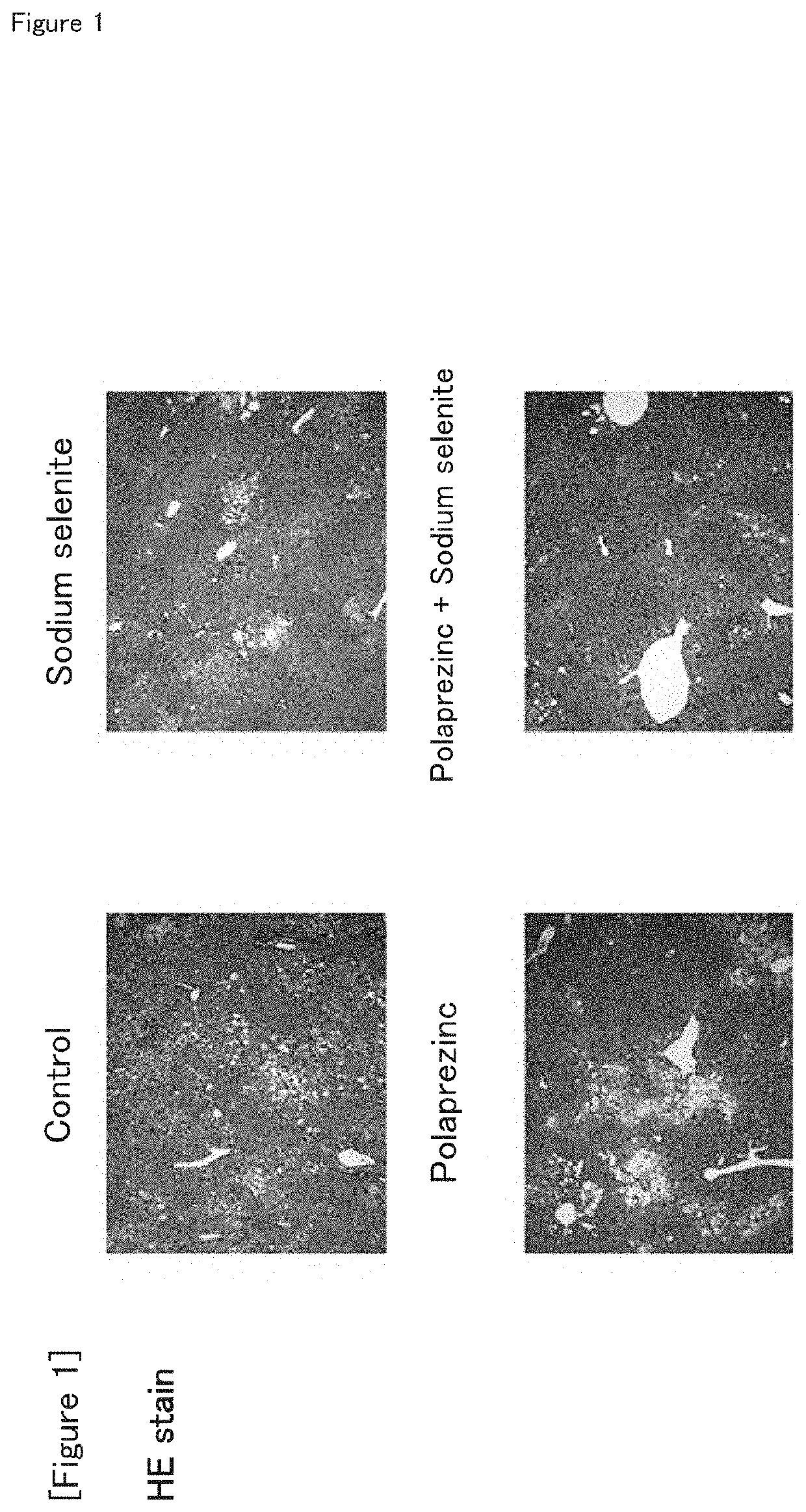
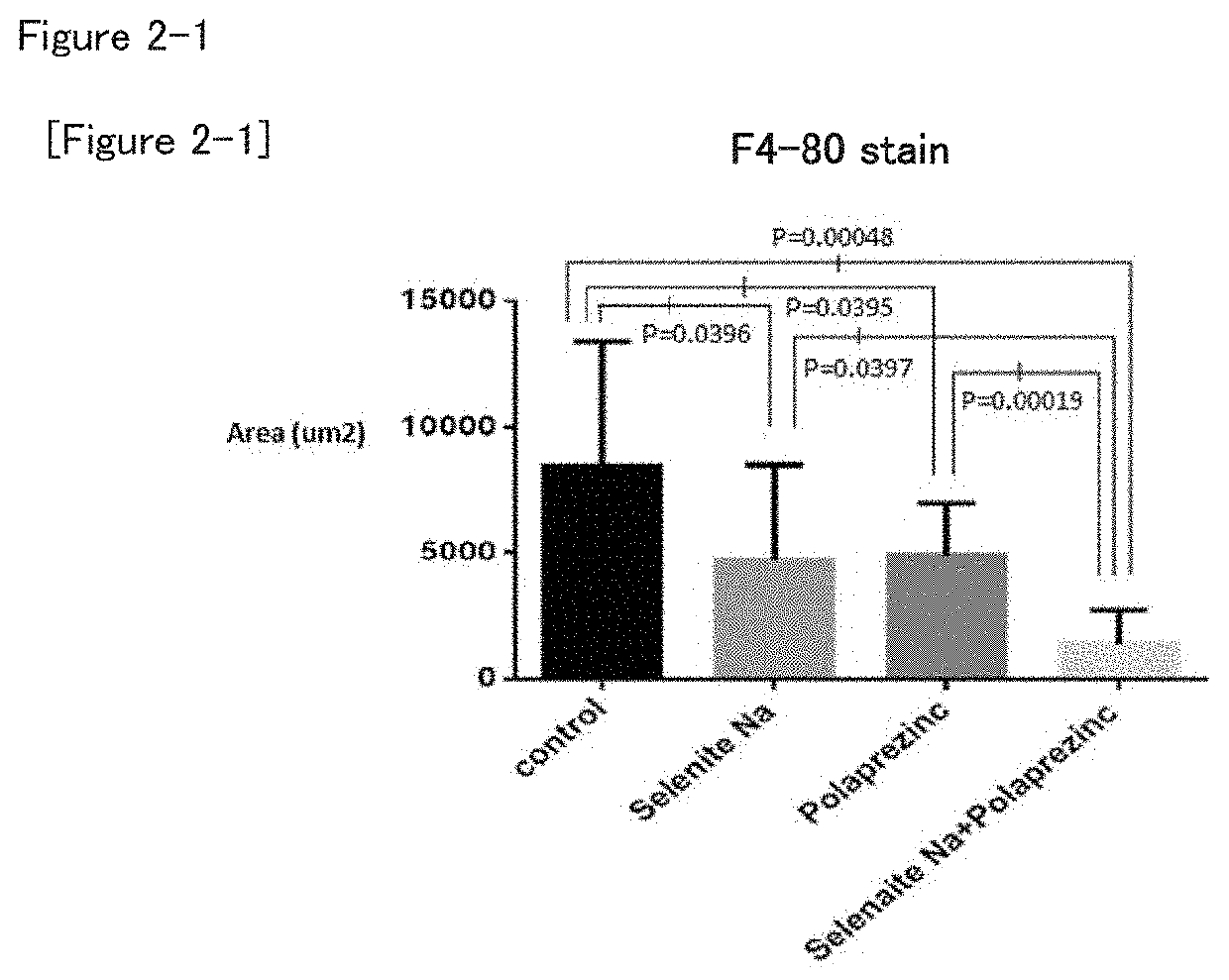
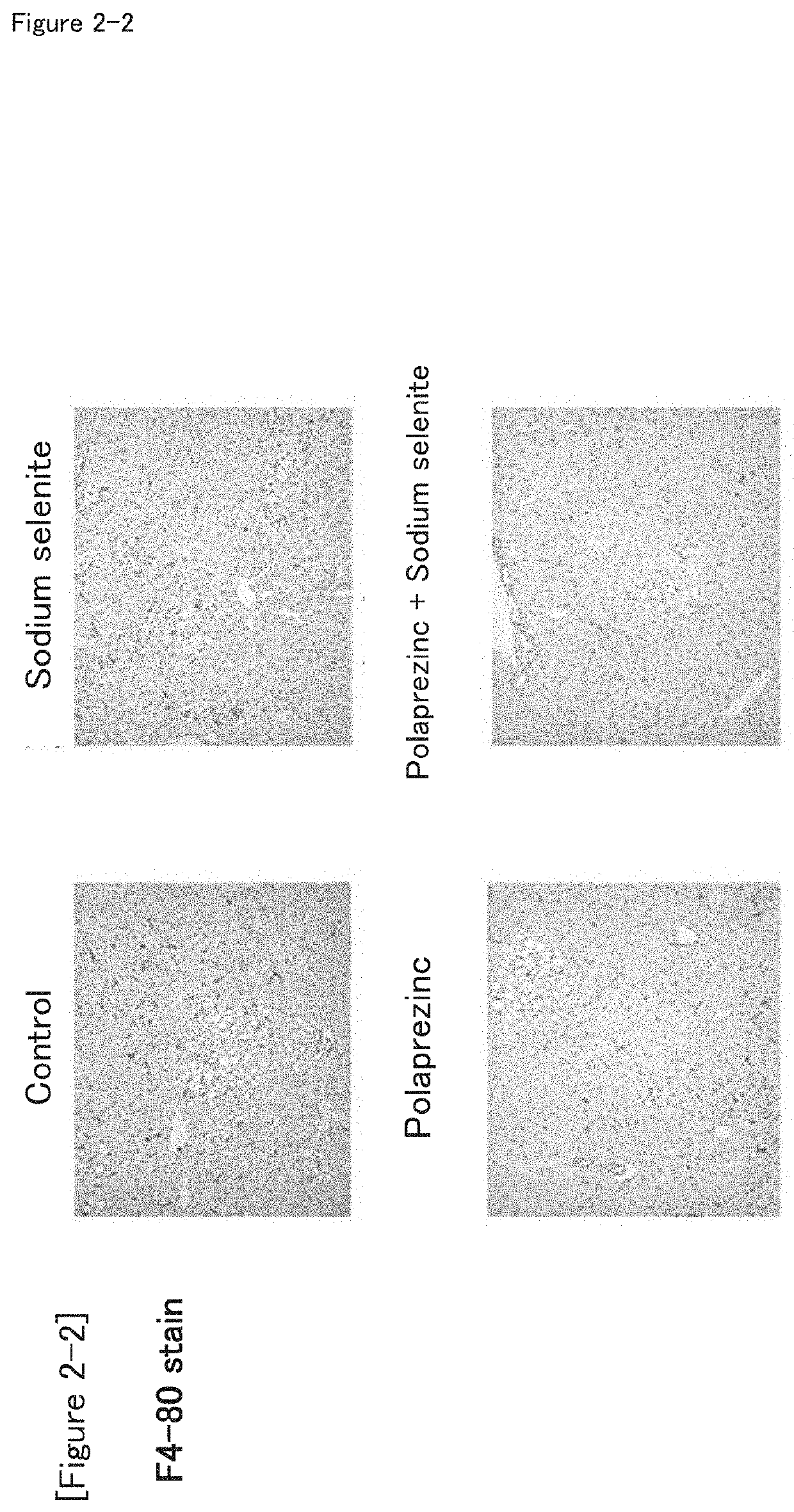
Combination drug suitable for treatment and prevention of non-alcoholic fatty liver disease (NAFLD) and/or non-alcoholic steatohepatitis (NASH), and/or hepatic fatty degeneration
a combination drug and fatty liver disease technology, applied in the direction of pharmaceutical delivery mechanism, drug composition, organic active ingredients, etc., can solve the problems of macrovesicular fat accumulation improvement, suppress the progression of cirrhosis, and improve the non-alcoholic fatty liver disease (nafld). the effect of normal liver recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0062]The NASH model mice were assigned to four groups (eight mice per group) by body weight stratification random sampling method so that the average body weight is equal. The four groups are the zinc preparation (polaprezinc)+selenium preparation (sodium selenite) group, zinc preparation (polaprezinc) alone group, selenium preparation (sodium selenite) alone group, and control group. The route of administration was oral administration, and the period of administration was 28 days.
[0063]The number of administration was once a day. The dosage was 10 ml / kg body weight and was administrated using an oral zonde.
[0064]The dosages of each group are shown below.
TABLE 1NumberDoseofTest groupDosageConcentration(ml / kg)animalsControl00108Selenium prepara-150μg / kg15μg / ml108tion (sodiumselenite)Zinc preparation45.2mg / kg4.52mg / ml108(polap rezinc)Zinc preparation45.2 mg / kg +4.52 mg / ml +108(polaprezinc) +150 μg / kg15 μg / mlSelenium prepara-tion (sodiumselenite)
[0065]Blood was collected from the hear...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com