Dry powder ketamine composition for use in the treatment of depression by pulmonary administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0114]
ComponentAmount (mg / unit)Esketamine hydrochloride3.45 (corresponds to2.99 mg esketamine)Lactose monohydrate LH200 LP19.16Magnesium stearate0.39
example 2
[0115]
ComponentAmount (mg / unit)Esketamine hydrochloride4.61 (corresponds to4 mg esketamine)Lactose monohydrate LH200 LP18.20Magnesium stearate0.18
example 3
[0116]
ComponentAmount (mg / unit)Esketamine hydrochloride5.06 (corresponds to4.39 mg esketamine)Lactose monohydrate LH200 LP17.581Magnesium stearate0.359
[0117]The compositions have been found uniform in accordance with requirements of Ph.Eur.2.9.40. Average esketamine hydrochloride content (n=10) was in the range 92.5%-107.5% of nominal dose.
[0118]The process has been found scalable to the scale of 1.8 kg.
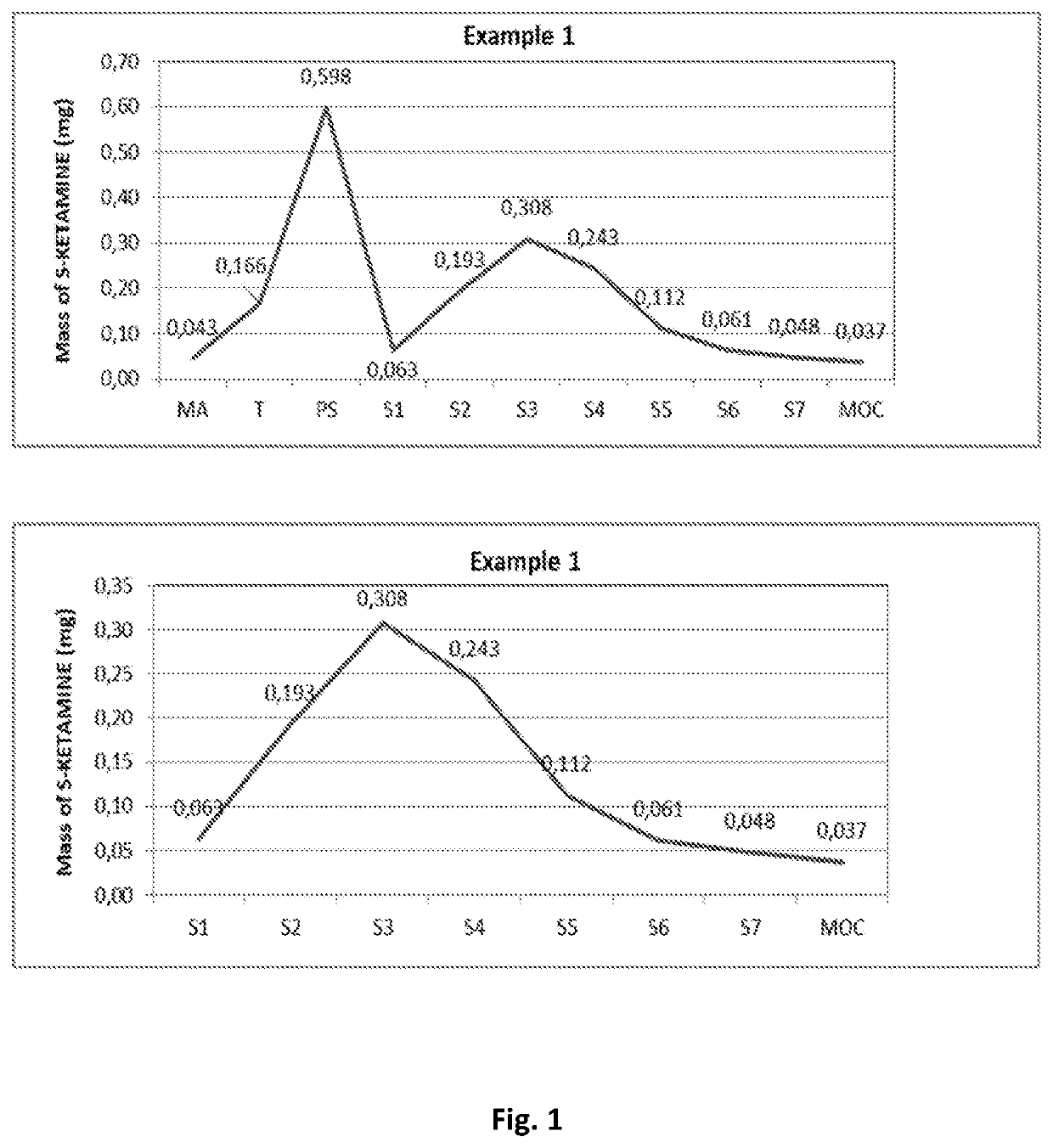
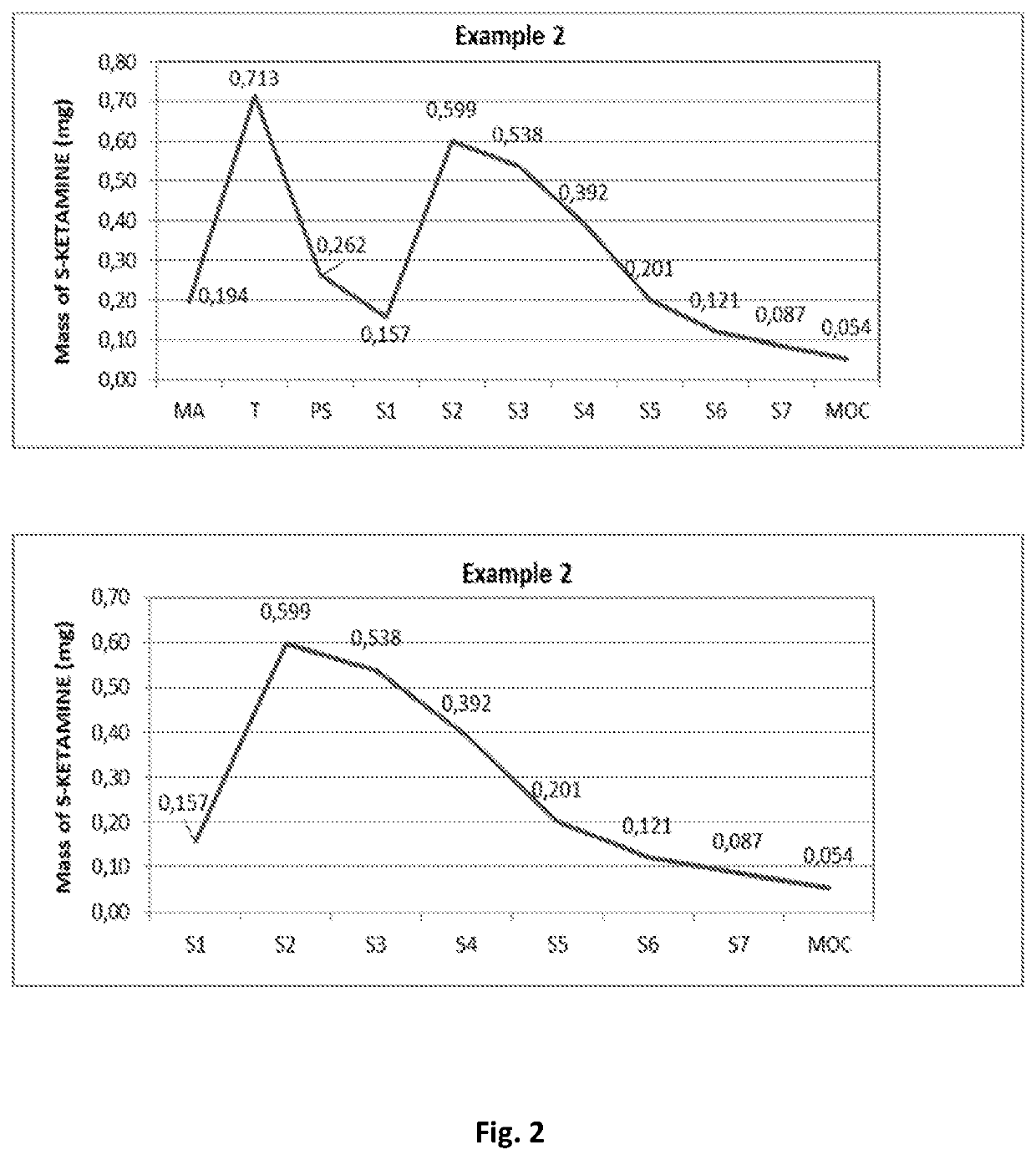
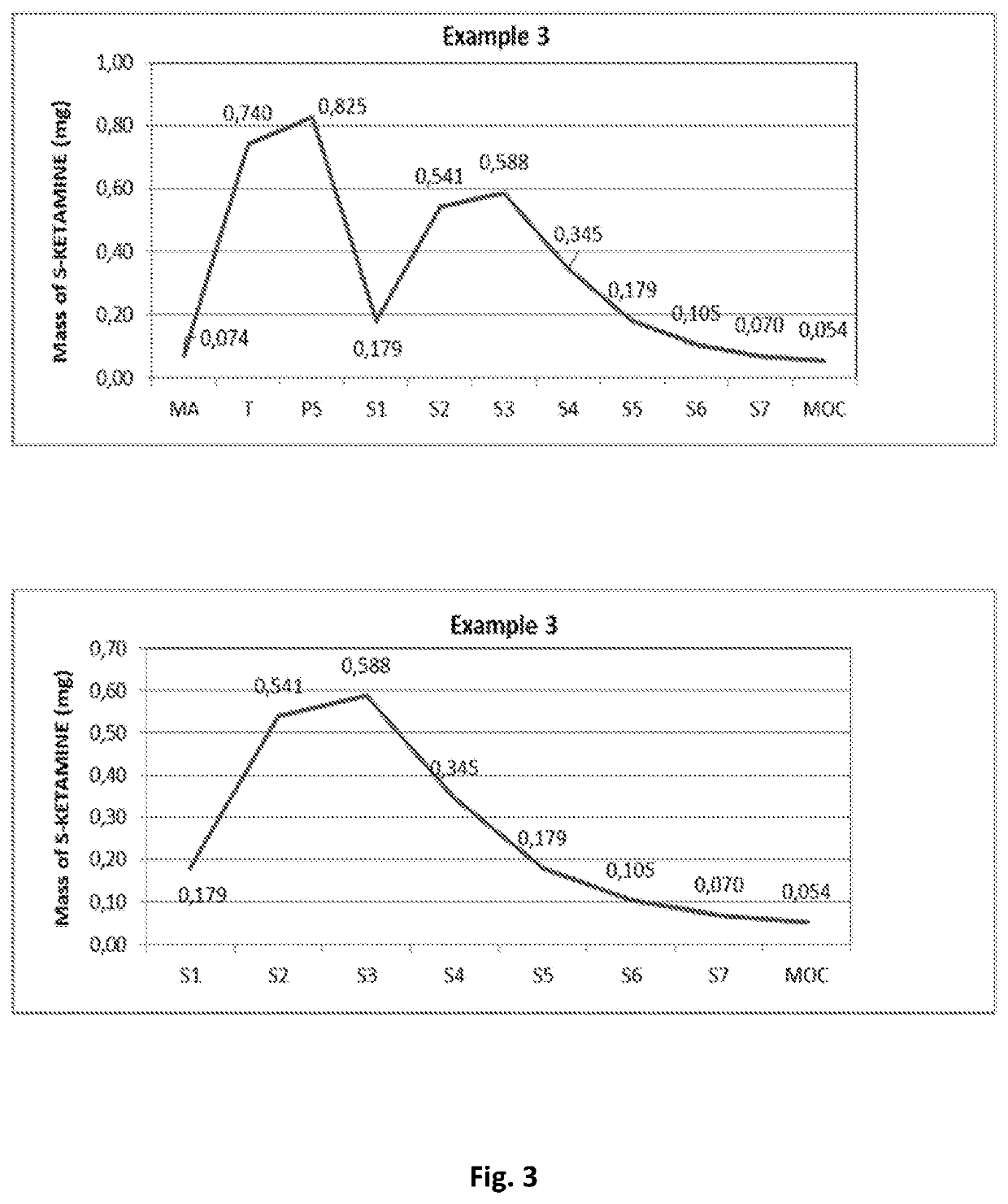
[0119]Aerodynamic Particle Size Distribution (APSD) test of the compositions of the Examples 1, 2 and 3 of the invention.
[0120]The compositions of Examples 1, 2 and 3 of the invention have been tested using the Next Generation Pharmaceutical Impactor (NGI) (Ph. Eur. Apparatus E) in accordance with the procedure for powder inhalers.
[0121]The results of the tests are presented in Table 1 below and in FIG. 1 (Example 1), FIG. 2 (Example 2) and FIG. 3 (Example 3) of the drawing, wherein upper diagrams present APSD data for the whole NGI and bottom diagrams present APSD data for stages 1-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com