Composition for Promoting Increase in Subcutaneous Tissue and Subcutaneous Adipose Tissue
a technology for subcutaneous tissue and adipose tissue, which is applied in the direction of prosthesis, drug composition, peptide/protein ingredients, etc., can solve the problems of necrosis of tissue, inability to use, and inability to find, so as to avoid induration, avoid bag rupture, and promote the effect of subcutaneous tissue increas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Composition for Breast Augmentation that Includes a Composition Promoting an Increase in Subcutaneous Tissue
[0066]For blood collection, 2.5 mL of heparin sodium (10 units / mL) was added to a syringe having a syringe volume of 50 cc and 50 mL of blood was collected. In total, 220 to 300 mL of blood was collected. The collected blood was centrifuged (a combination of KUBOTA 2420 and KUBOTA RS-240 (a rotor), at 4,000 rpm for 10 minutes) to separate plasma.
[0067]25 mL of the separated plasma was collected in a 50 cc syringe, thereby obtaining heparinized plasma.
[0068]In order to obtain heated autologous plasma, after the heparinized plasma was obtained, the heparinized plasma was subjected to a heat treatment of the plasma at 100° C. for 10 minutes by using a dry thermo unit (Dry Thermo Unit DTU-1C by TAITEC Corporation) and subsequently, the plasma was cooled rapidly. Consequently, heated autologous plasma in a gel form was obtained.
[0069]A Trafermin (registered tradema...
example 2
A Specific Example of Administration of a Composition for Breast Augmentation that Includes a Composition Promoting an Increase in Subcutaneous Tissue (Case 1)
[0074]The composition for breast augmentation of the present invention was administered to a 45-year-old woman to whom sufficient informed consent was given in advance and who gave consent, and the effect of breast augmentation was evaluated.
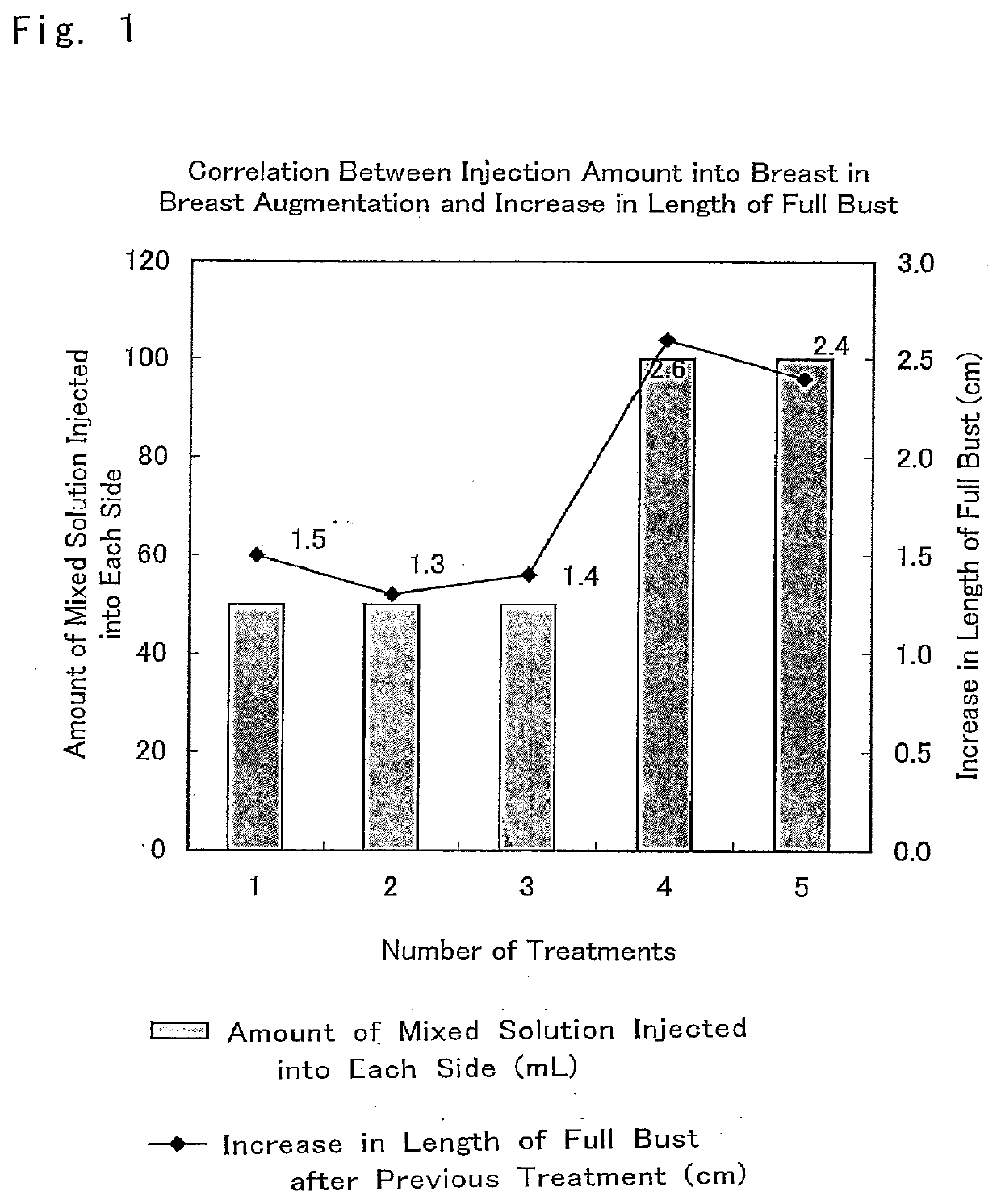
[0075]The composition for breast augmentation that included the composition promoting an increase in subcutaneous tissue prepared according to the above-described Example 1 was used. A mixed solution of Trafermin, Intralipid (a lipid emulsion), and the heparinized autologous plasma was administered between the mammary gland and the pectoralis major fascia five times.
[0076]In the first to the third administrations, 50 mL for each side, that is, 100 mL in total was administered. In the fourth and the fifth administrations, 100 mL for each side, that is, 200 mL in total was administered. The ...
example 3
A Specific Example of Administration of a Composition for Breast Augmentation that Includes a Composition Promoting an Increase in Subcutaneous Tissue (Case 2)
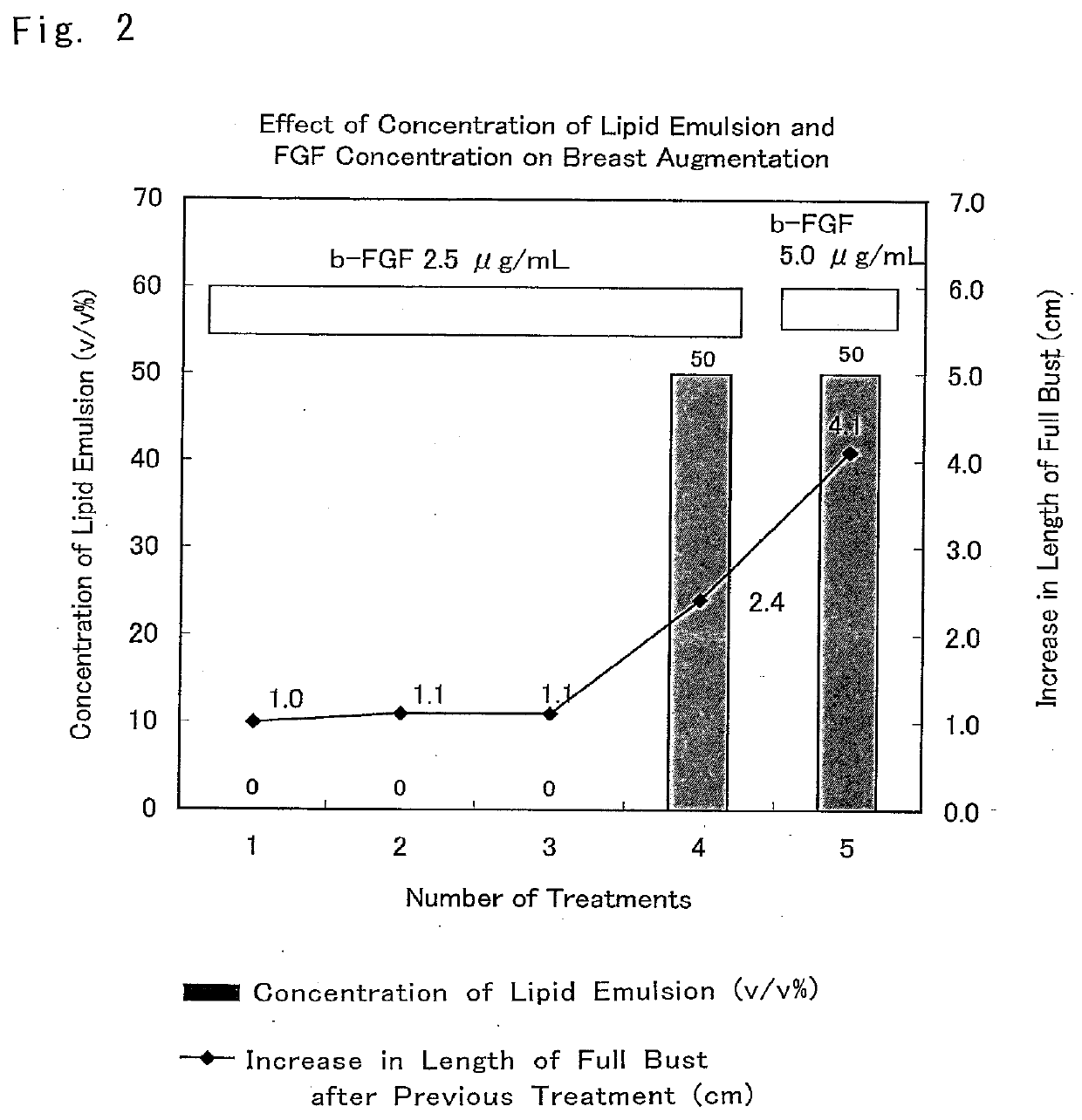
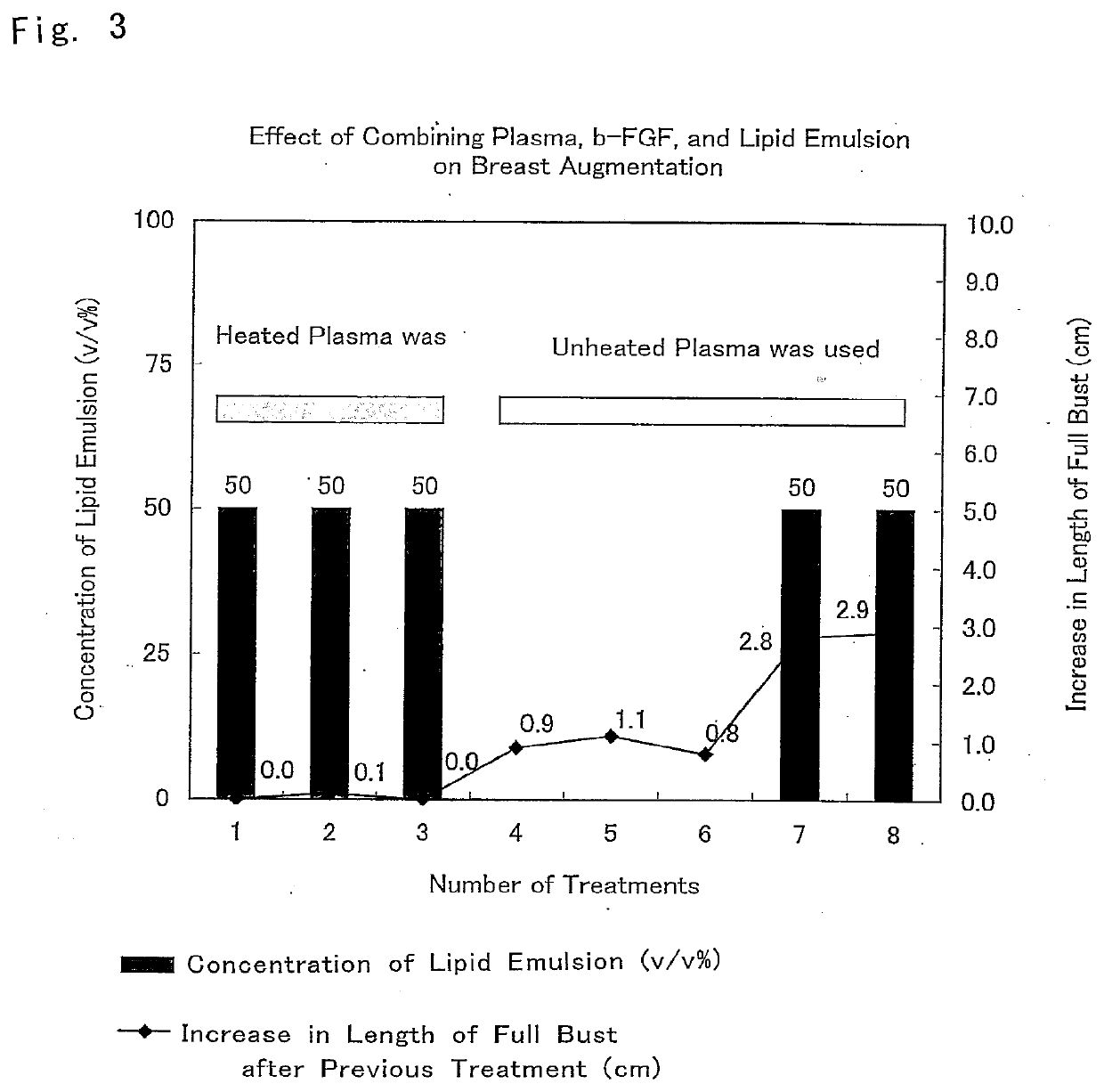
[0080]The composition for breast augmentation of the present invention was administered to a 36-year-old woman to whom sufficient informed consent was given in advance and who gave consent, and the effect of breast augmentation was evaluated.
[0081]First, it was determined which factor was necessary in a mixed solution of Trafermin, a lipid emulsion (Intralipid), and heparinized autologous plasma that constituted the composition for breast augmentation of the present invention.
[0082]Initially, the composition for breast augmentation that included the composition promoting an increase in subcutaneous tissue of the present invention, the inventive composition being composed of a mixed solution of Trafermin and heparinized autologous plasma and including no lipid emulsion (Intralipid) was administered three times. Consequently, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com