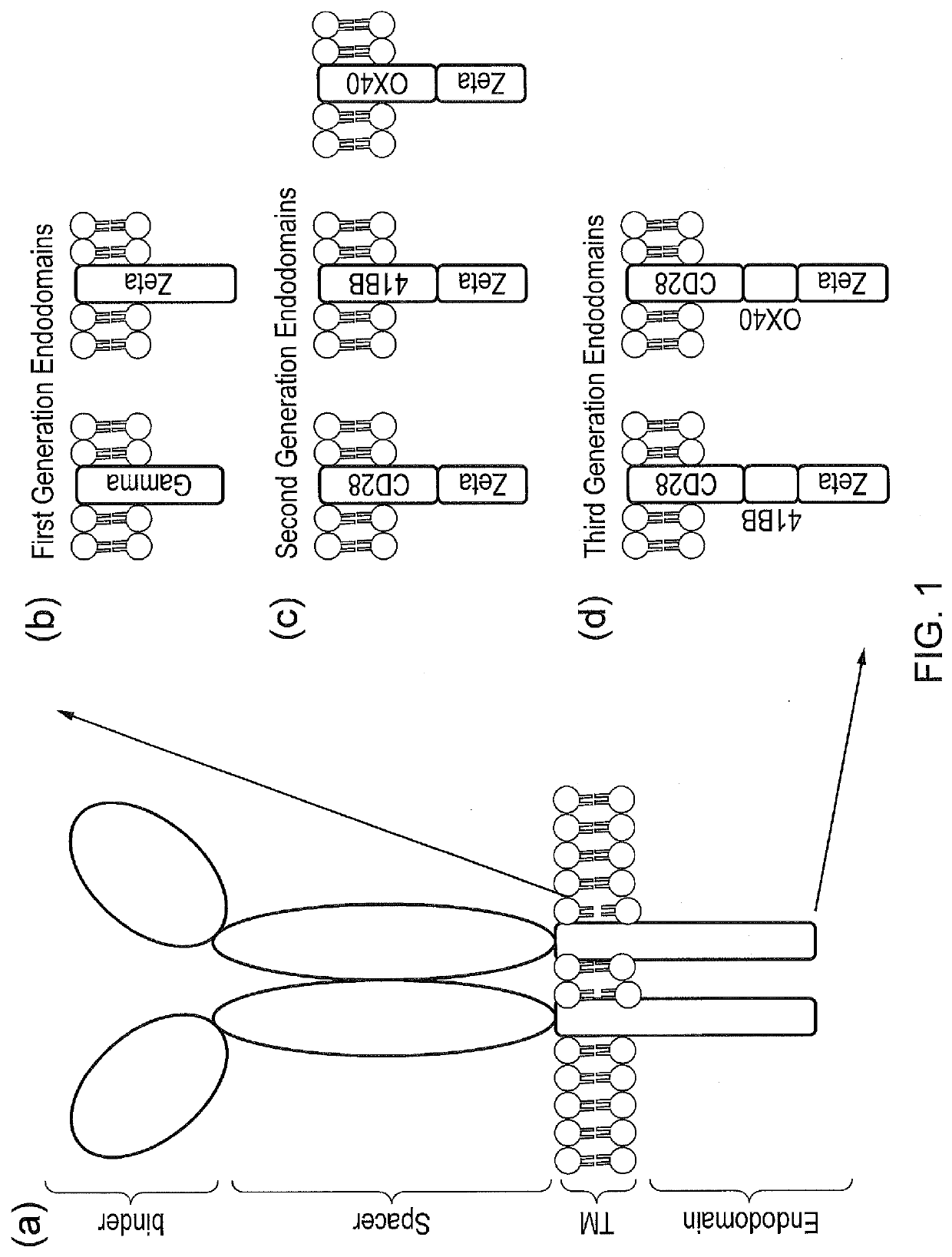
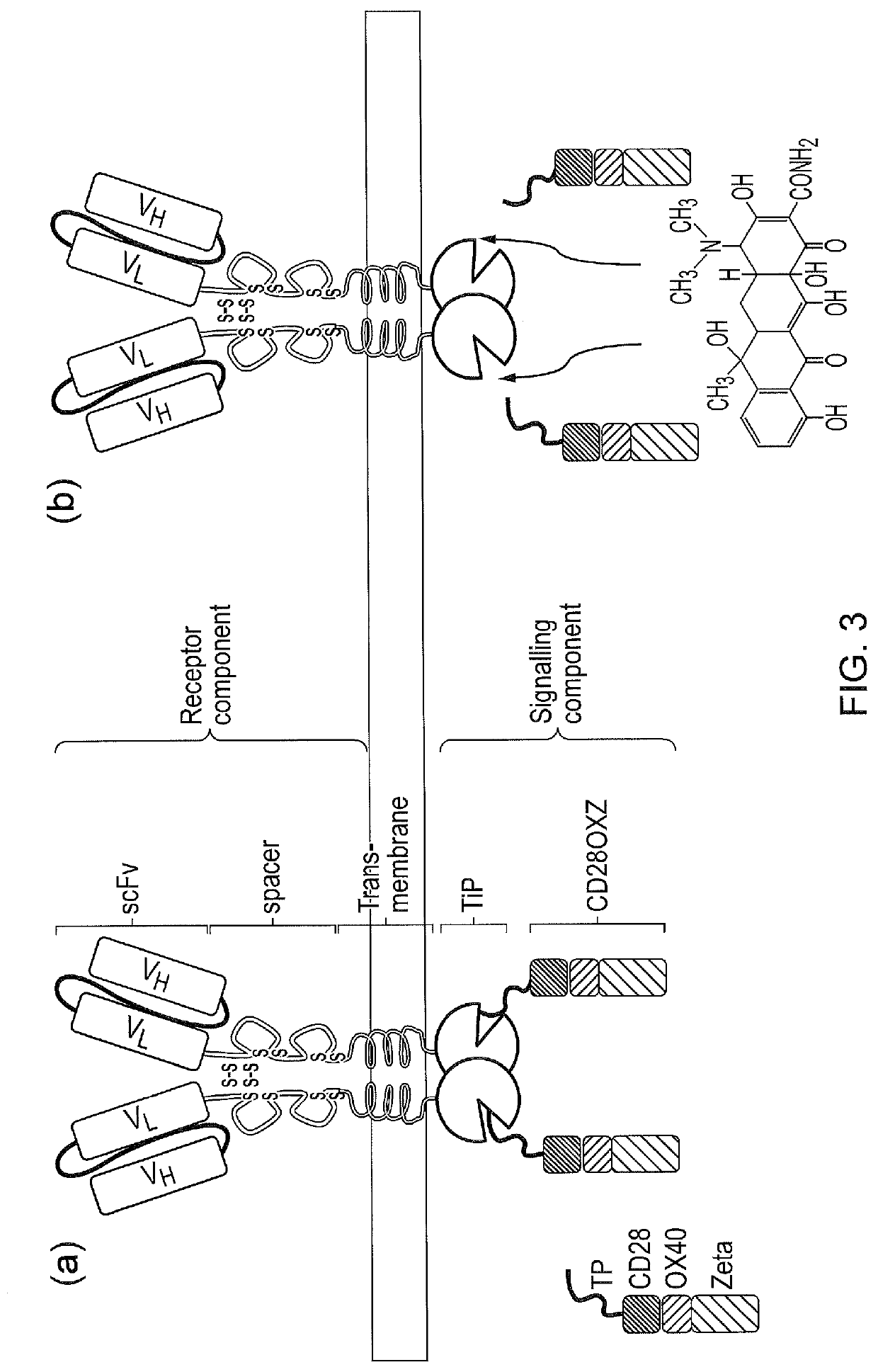
Signalling system
a signalling system and chimeric antigen technology, applied in the direction of peptides, drug compositions, genetically modified cells, etc., can solve the problems of “on-target off-tumour syndrome, difficult and quite often impossible to select and expand large numbers of t-cells specific for most cancer antigens, etc., and achieve the effect of rapid inhibition/termination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
lity of the TetCAR Signalling System
[0225]A bicistronic construct was expressed as a single transcript which self-cleaves at the 2A site to yield TiP fused to eGFP and a CAR with TetR as its endodomain (FIG. 5a).
[0226]Fluorescent microscopy of SupT1 cells expressing this construct in the absence of tetracycline demonstrated that eGFP fluorescence can clearly be seen at the cell membrane (FIG. 5b); whilst in the presence of tetracycline the eGFP was cytoplasmic (FIG. 5c). These data demonstrate that tetracycline has displaced TiP from the TetR CAR.
example 2
g Through the TetCAR System
[0227]A bicistronic construct was expressed in BW5 T cells as a single transcript which self-cleaves at the 2A site to yield a signalling component which comprises TiP fused via a flexible linker to the endodomain of CD3-Zeta; and a receptor component which comprises a CD33 recognizing scFv, a spacer derived from the Fc domain of IgG1, a CD4 derived transmembrane and intracellular domain; and TetR (FIG. 6a). A control was also expressed which was identical except that TiP was absent from the signalling component (FIG. 6b).
[0228]The BW5 T-cells were challenged with wild-type SupT1 cells or SupT1 cells engineered to express CD33 in the absence of tetracycline or in the presence of increasing concentrations of tetracycline. T-cells challenged with wild-type SupT1 cells did not activate in either the presence or absence of Tetracyline; T-cells challenged with SupT1 cells expressing CD33 were activated in the absence of Tetracycline, but activation is rapidly i...
example 3
g of the TetCAR System in Primary T Cells
[0230]SupT1 cells (which are CD19 negative), were engineered to be CD19 positive giving target negative and positive cell lines which were as similar as possible. Primary human T-cells from 3 donors were transduced with three CAR constructs: (i) “Classical” 1st generation anti-CD19 CAR; (ii) 1st generation anti-CD19 tetCAR; (iii) Control anti-CD19 tetCAR where TiP is missing from endodomain. Non-transduced T-cells and T-cells transduced with the different CAR constructs were challenged 1:1 with either SupT1 cells or SupT1.CD19 cells in the presence of different concentrations of Tetracycline. Supernatant was sampled 48 hours after challenge. Supernatant from background (T-cells alone), and maximum (T-cells stimulated with PMA / Ionomycin) was also samples. Interferon-gamma was measured in supernatants by ELISA (FIG. 13). “Classical” CAR T-cells were activated by SupT1.CD19 irrespective of tetracycline. TetCAR T-cell were activated by SupT1.CD19...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com