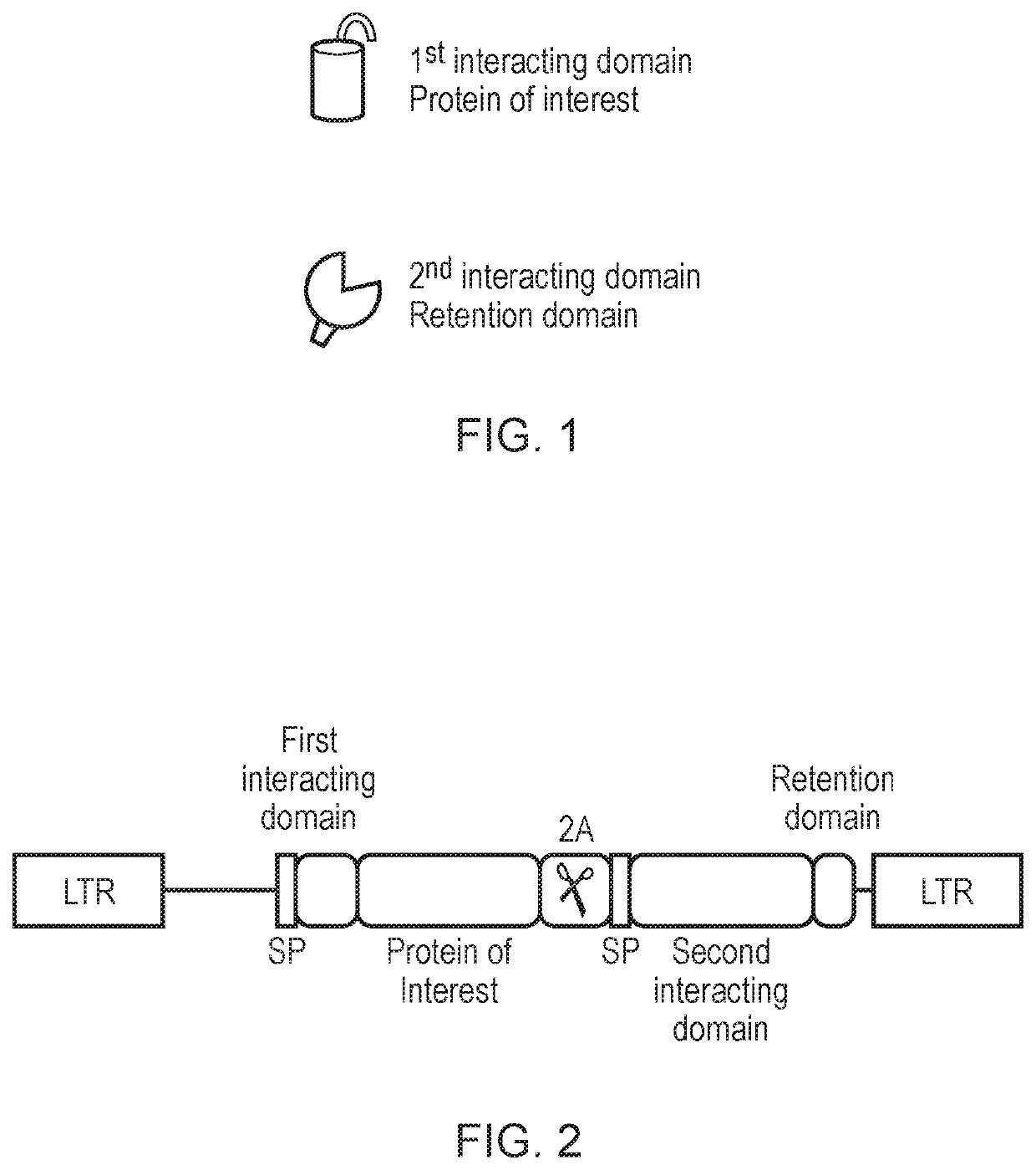
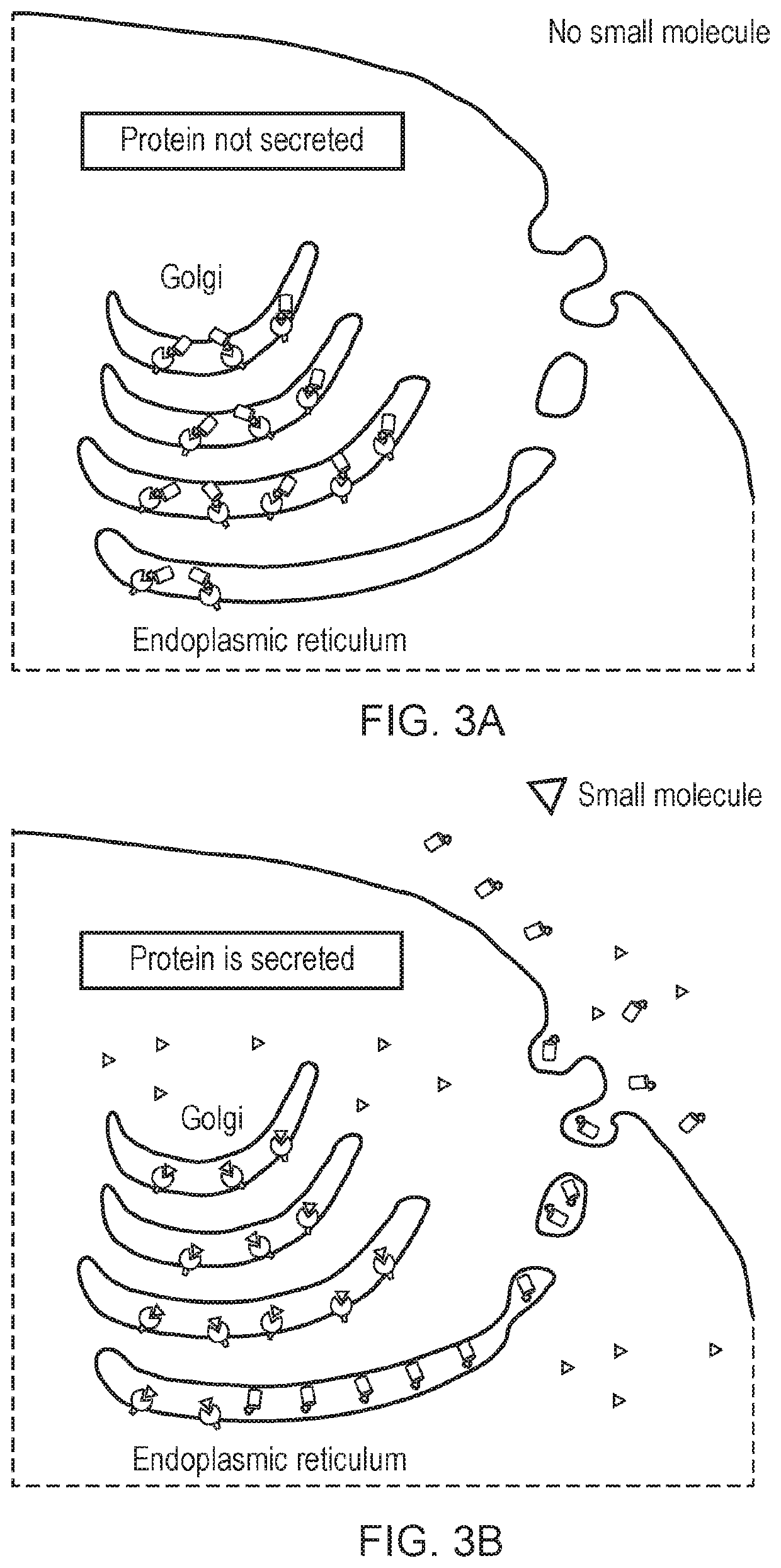
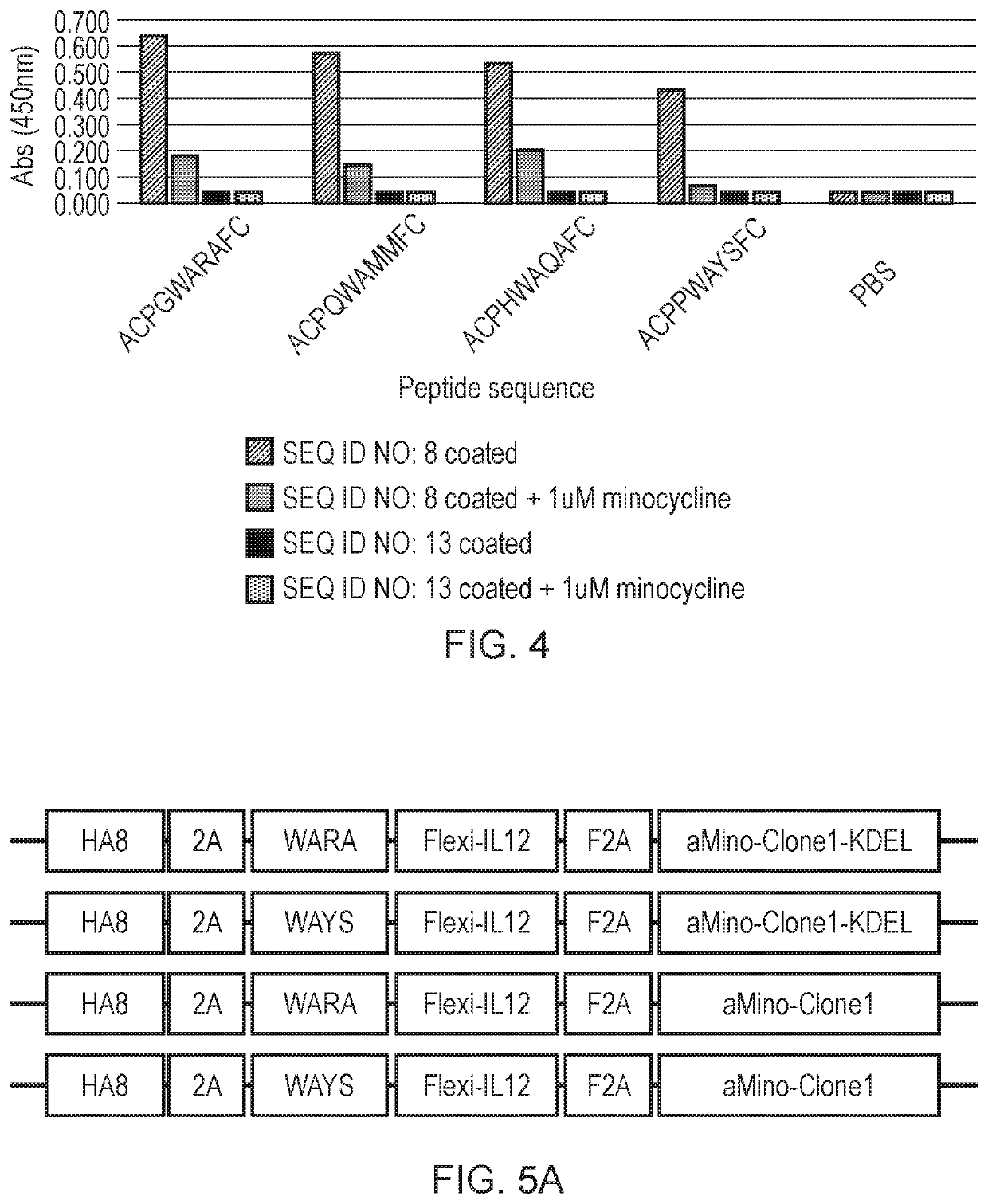
Engineered cytolytic immunecell
a technology of cytolytic immune cells and cytolytic antibodies, applied in the direction of blood/immune system cells, peptides, drug compositions, etc., to achieve the effect of increasing anti-tumour effects and reducing adverse toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of Peptides which Bind to Minocycline dAb
[0404]A minocycline dAb-Fc was immobilised on beads. The minocycline dAb was encoded by SEQ ID NO: 8. Phage-peptide colony screening was performed with and without 1 μM minocycline.
[0405]Peptide library panning identified peptides with displaceable specificity for a minocycline dAb encoded by SEQ ID NO: 8. An antibody comprising SEQ ID NO: 13 was used as a control antibody (the control antibody comprises different CDRs to SEQ ID NO: 8).
[0406]Four peptides were identified with displaceable specificity for minocycline dAb. The peptides are: ACPGWARAFC (SEQ ID NO: 34); ACPHWAQAFC (SEQ ID NO: 35); ACPQWAMMFC (SEQ ID NO: 36) and ACPPWAYSFC (SEQ ID NO: 37).
[0407]The results of binding experiments using each of these peptides are shown in FIG. 4.
example 2
d Secretion of IL12 by KDEL-Tagged dAb and Minocycline-Dissociating Peptide
[0408]A platform was generated consisting of a dAb with affinity for minocycline and several peptides that can bind to the dAb but will dissociate in the presence of minocycline (SEQ ID NO: 34-SEQ ID NO: 37).
[0409]The KDEL amino acid sequence was used to retain proteins within the ER / Golgi apparatus. The minocycline specific dAb was tagged with the KDEL sequence thus anchoring it within with the ER / Golgi and a molecule of interest (IL12) is in turn tagged with the peptide with affinity to the dAb causing its retention. Upon addition of minocycline, the dAb-peptide complex dissociates enabling secretion of the peptide-tagged molecule.
[0410]A set of constructs were designed to test this hypothesis using IL12 fused to the minocycline peptide mimic. IL12 is both a molecule of interest in its own right due its proliferative effect on T-cells and a convenient model of a secreted protein, which can be assayed by ELI...
example 3
Testing of Control of Secretion by Retention
[0422]T-cells are transduced to express a CD19 CAR, CD19CAR-2A-flexilL-12 (which provides constitutive expression of IL-12) and CD19CAR-2A-TiP-FlexilL12-2A-S-TetR-sekdel (which provides tuneable expression of IL-12). The T-cells are cultured in different concentrations of minocycline. Supernatant is harvested after 48 hours and IL-12 is quantified using an enzyme-linked immunosorbent assay (ELISA).
[0423]The functional properties of the different CAR T-cells are quantified using standard co-culture methods with CD19 targets and their function determined by flow-cytometry and cytokine release in the presence of different concentrations of minocycline.
PUM
| Property | Measurement | Unit |
|---|---|---|
| nucleic acid | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| plasma membrane retention | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com