Compositions and Methods for Cancer Immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
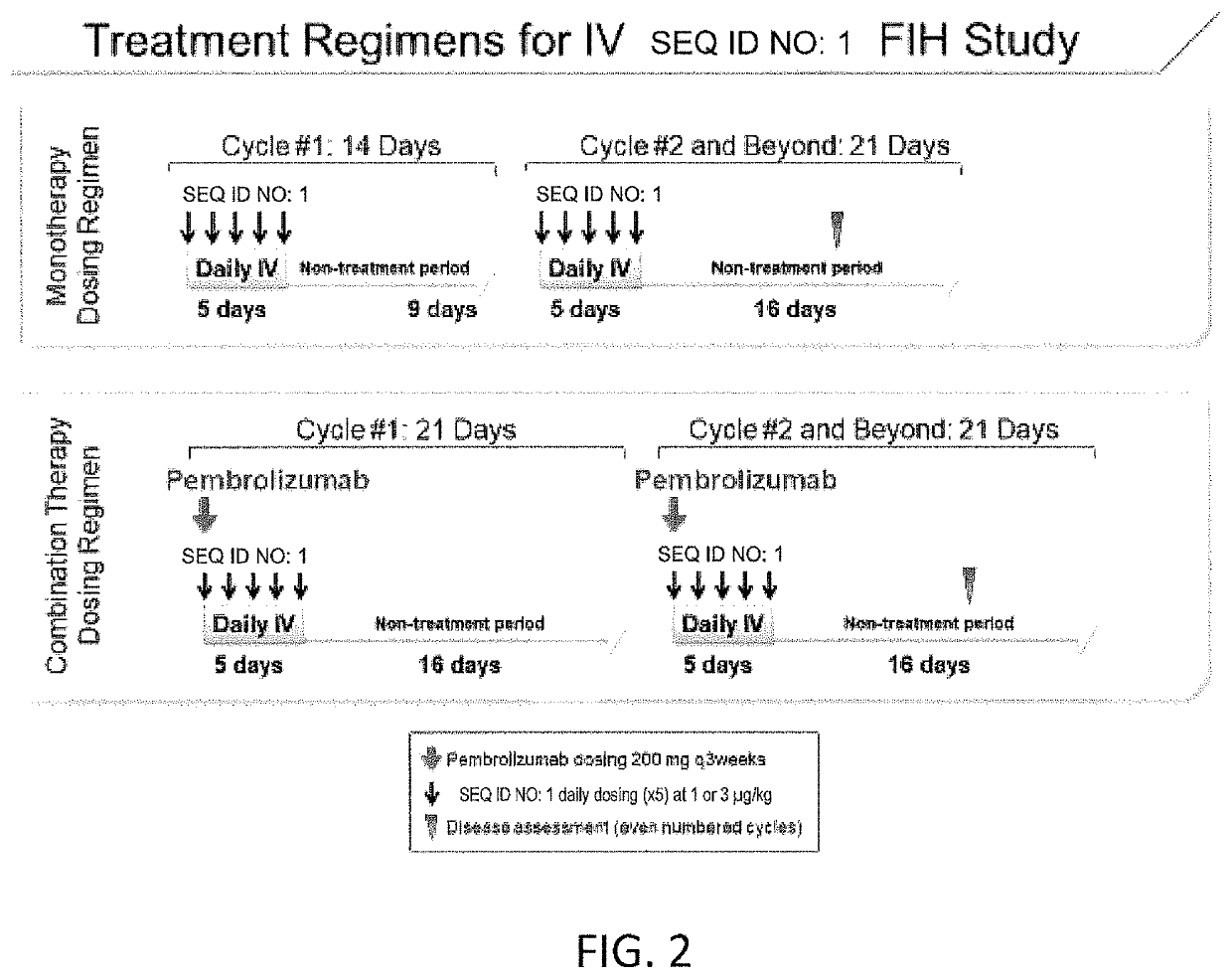
Study of the Fusion Protein of SEQ ID NO: 1 Administered Intravenously as Monotherapy and in Combination with Pembrolizumab in Subjects with Advanced Solid Tumors
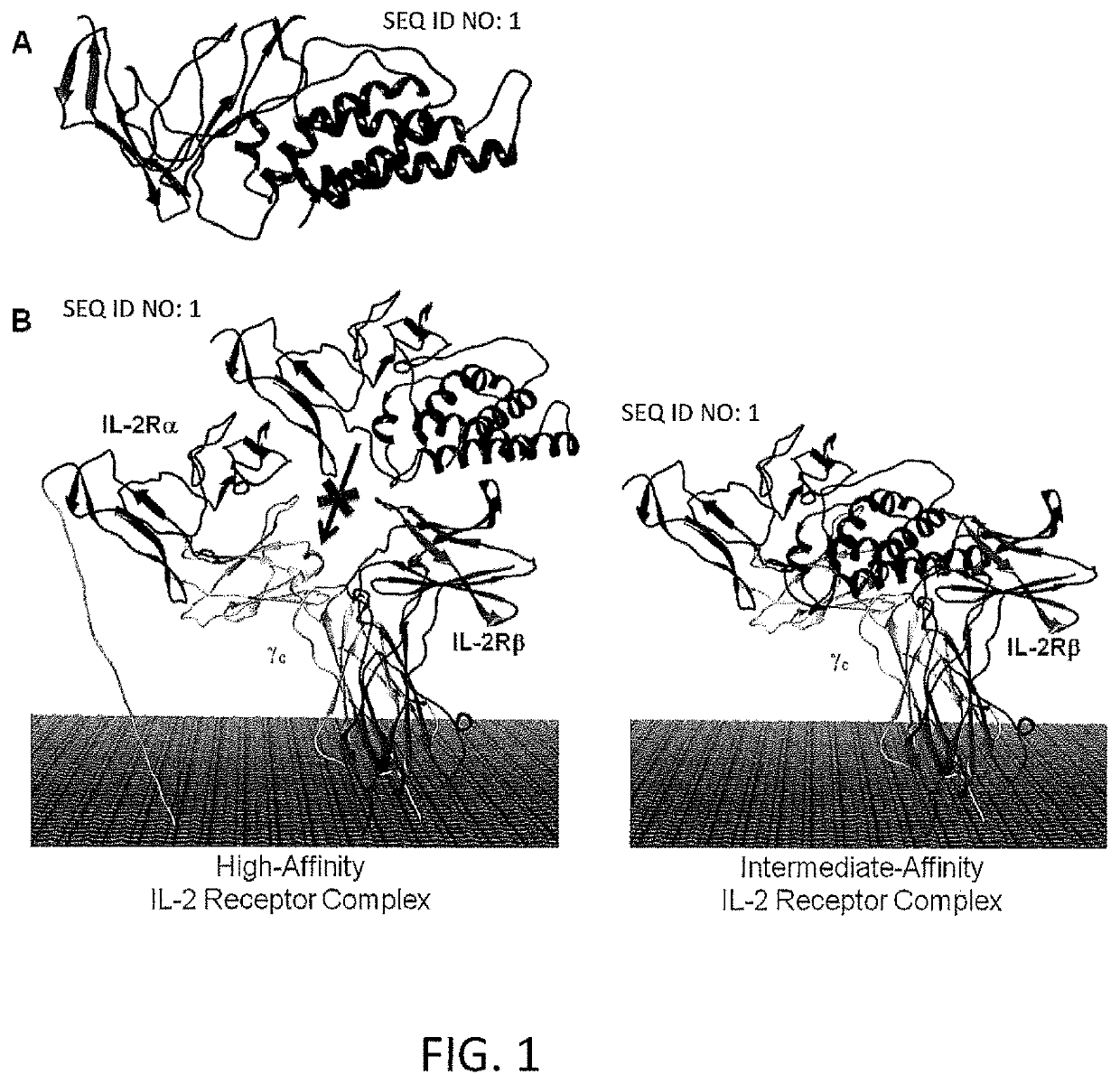
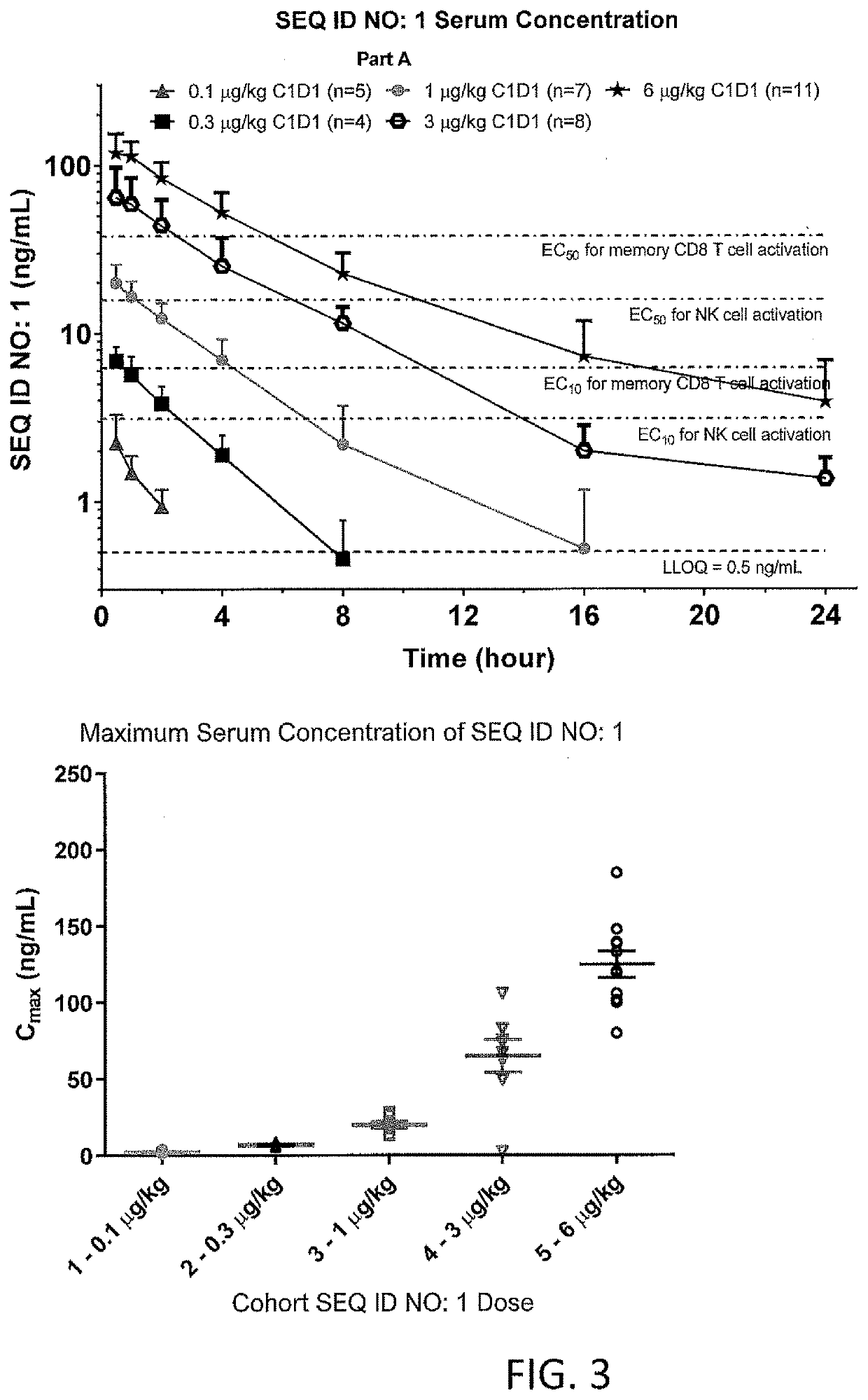
[0229]The Fusion Protein of SEQ ID NO: 1 is a fusion of circularly permuted IL-2 and IL-2 Receptor a (IL-2Rα) designed to selectively activate the intermediate-affinity IL-2R, comprised of IL-2Rβ and γ, for activation of cytotoxic CD8+ T cells and NK cells. The intermediate-affinity IL-2R is expressed predominantly on effector lymphocytes, which play an important role in driving antitumor immune responses. Wild-type IL-2 activates the high-affinity IL-2R, comprised of IL-2Rα, β, and γc, driving the expansion of immunosuppressive CD4+ regulatory T (Treg) cells at concentrations below those at which intermediate-affinity IL-2R-bearing effector cells are activated. Selective activation of the intermediate affinity IL-2R has the potential to enhance tumor killing and was shown to possess enhanced antitumor activity relative to ...
example 2-peripheral
Blood Lymphocyte Responses in Patients with Renal Cell Carcinoma (RCC) Treated with High Dose IL-2
Background
[0254]Recombinant human interleukin-2 (rhlL-2, aldesleukin) is approved and used for the treatment of metastatic melanoma and renal cell carcinoma.1-8 However, the use of rhlL-2 is limited to patients with normal cardiac and pulmonary function due to associated capillary leak syndrome and resulting hypotension.9-12
[0255]Despite the poor tolerability associated with rhlL-2 treatment, it remains one of the few treatment regimens for metastatic melanoma and renal cell carcinoma that elicits a complete and durable response in a subset of patients, up to 12% in melanoma and 7% in renal cell carcinoma.7,8 It has been hypothesized that rhlL-2 preferentially activates and induces the expansion of immunosuppressive CD4+ Tregs,13 and high-dose IL-2 is required to induce signaling on receptor complexes expressed on potential tumor killing CD8+ T cells and natural killer (NK) cells.
[0256...
example 5 -
Example 5-Continuation of Clinical Trial Study, Part a of Phase 1 / 2 Expansion Dose to 8 μg / kg / day
[0307]
List of Abbreviations:Abbreviationor TermExplanation or DefinitionAEadverse eventANCabsolute neutrophil countC2D15Cycle 2, Day 15CDcluster of differentiationCIconfidence intervalCmaxmaximum drug concentration in serumCRcomplete responseCSAClinical Study AgreementCTCAECommon Terminology Criteria for Adverse EventsDCRdisease control rateDLTdose-limiting toxicityDORduration of responseECGstandard 12-lead electrocardiogramECOGEastern Cooperative Oncology GroupeCRFelectronic case report formEOTend of treatmentFIHfirst-in-humanGCPGood Clinical PracticeGLPGood Laboratory Practicei-immune-iAEimmune adverse eventICFinformed consent formICHInternational Council for HarmonisationiCRimmune complete responseiDCRimmune disease control rateiDORimmune duration of responseIFNinterferonIL-2interleukin-2IL-2Rinterleukin-2 receptoriORRimmune overall response rateiPDimmune progressive diseaseiPFSimmune...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com