Scaffolded fusion polypeptides and compositions and methods for making the same
a technology of fusion polypeptides and compositions, applied in the direction of peptides, polypeptides with his-tags, dna/rna fragmentation, etc., can solve the problems of insufficient method for expressing the soluble portions of many membrane proteins, inability to obtain large quantities of integrated membrane proteins, and inability to accurately determine the useful dose, so as to inhibit the growth or spread of viral infections. , the effect of reducing the number of viral infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
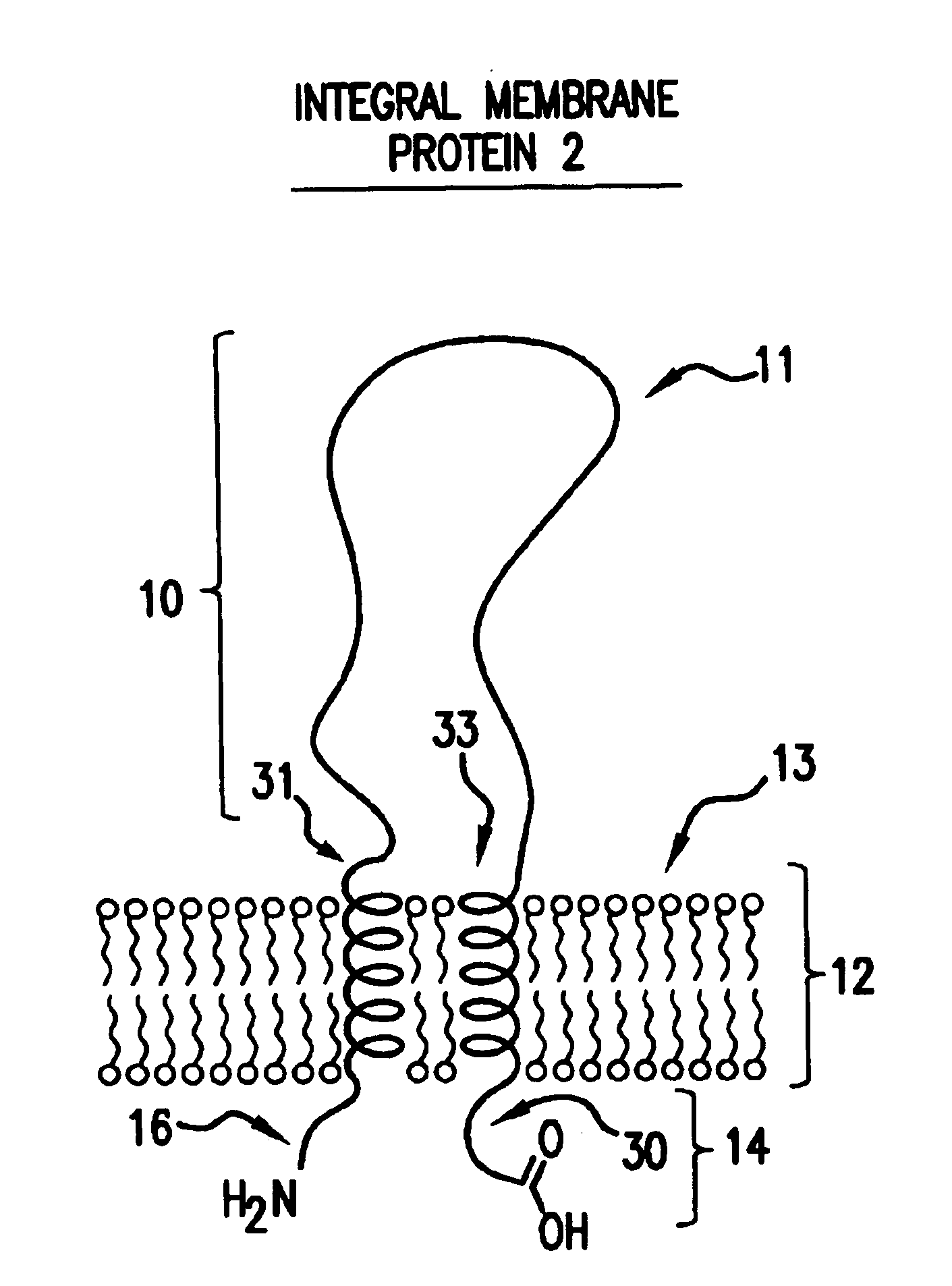
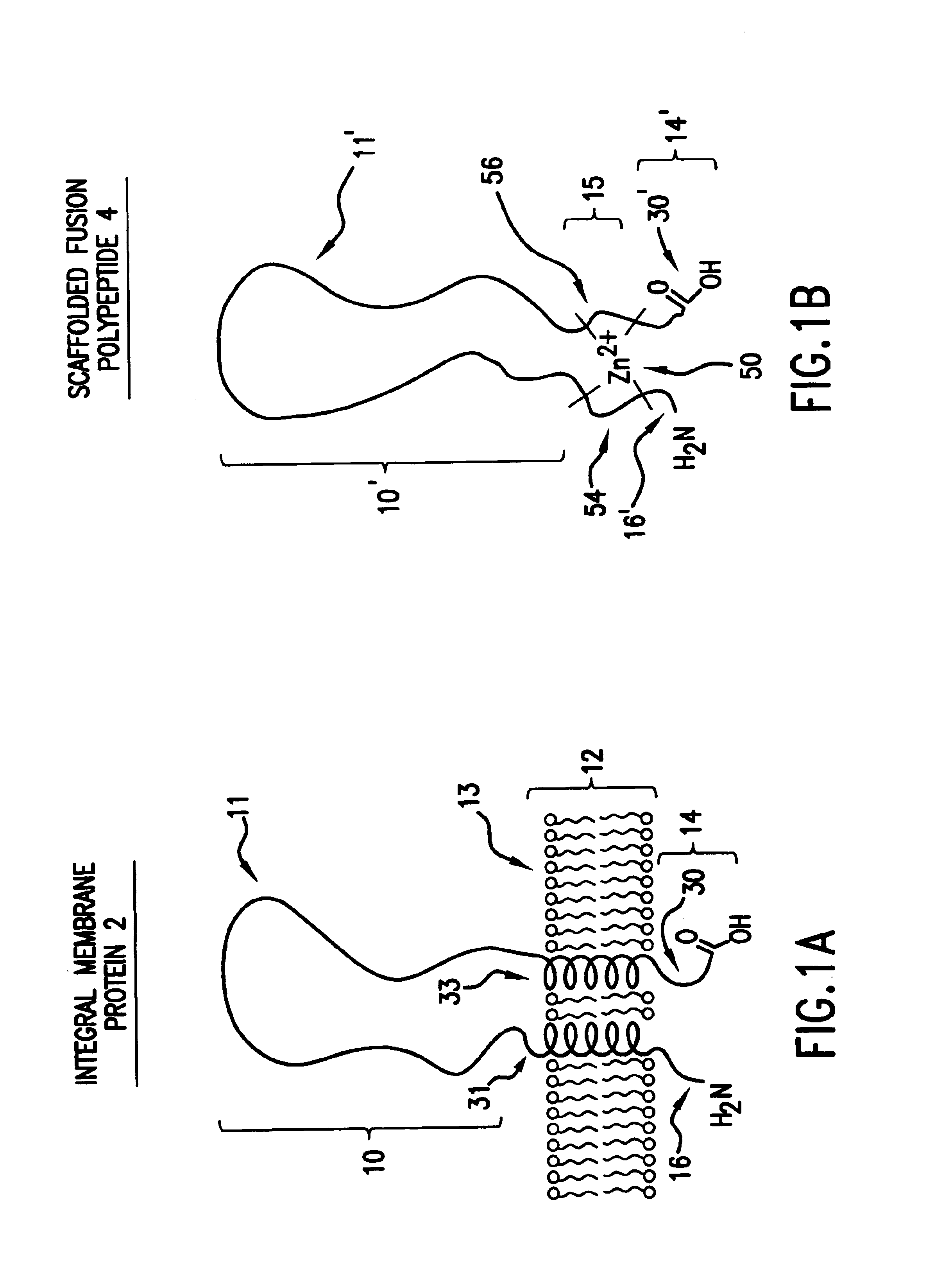
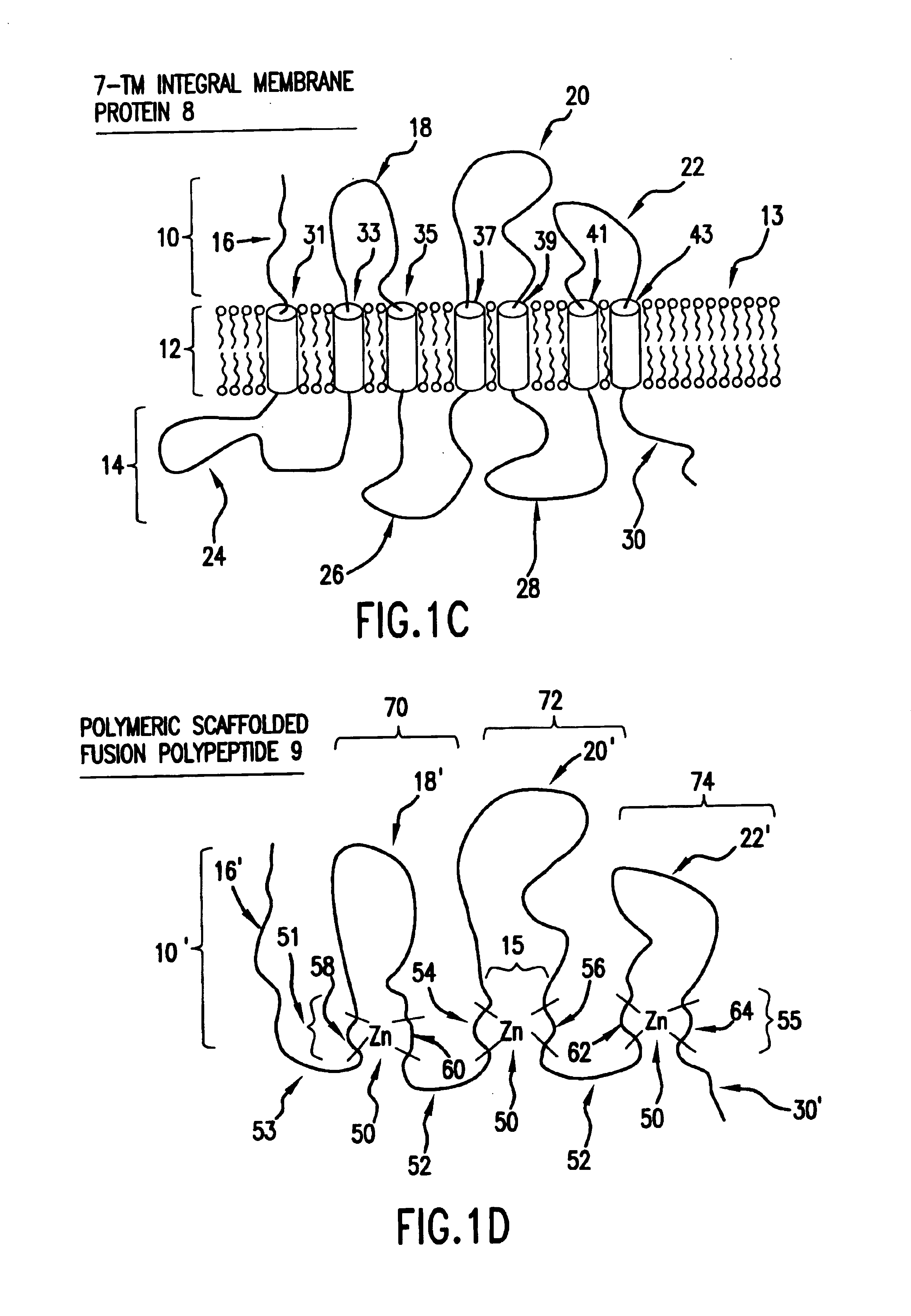
As discussed in the Background section, to date there are no known generally applicable methods which permit the isolation, synthesis or expression of integral membrane proteins, or domains of interest thereof, that provide high yield while retaining relevant structure and activity.
The present invention addresses these and other shortcomings in the art by providing novel compositions designed from integral membrane proteins and that mimic or possess one or more of the biological activities of integral membrane proteins from which they were designed. Moreover, in many instances compositions will exhibit improved solubility in cell-free and detergent-free aqueous media compared to the integral membrane protein from which they were designed.
5.1 Abbreviations
The amino acid notations used herein for the twenty genetically encoded L-amino acids are conventional and are as follows:
As used herein, unless specifically delineated otherwise, the three-letter amino acid abbreviations designate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| soluble | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com