Biocompatible tissue for therapeutic use and method of making same
a biocompatible tissue and tissue technology, applied in the field of biocompatible graft material and a method of making the same, can solve the problems of incomplete rejection of the graft material itself, high cost, and complex methods, and achieve the effect of conserving body tissu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0016]Although the disclosure hereof is detailed and exact to enable those skilled in the art to practice the invention, the physical embodiments herein disclosed merely exemplify the invention which may be embodied in other specific structure. While the preferred embodiment has been described, the details may be changed without departing from the invention, which is defined by the claims.
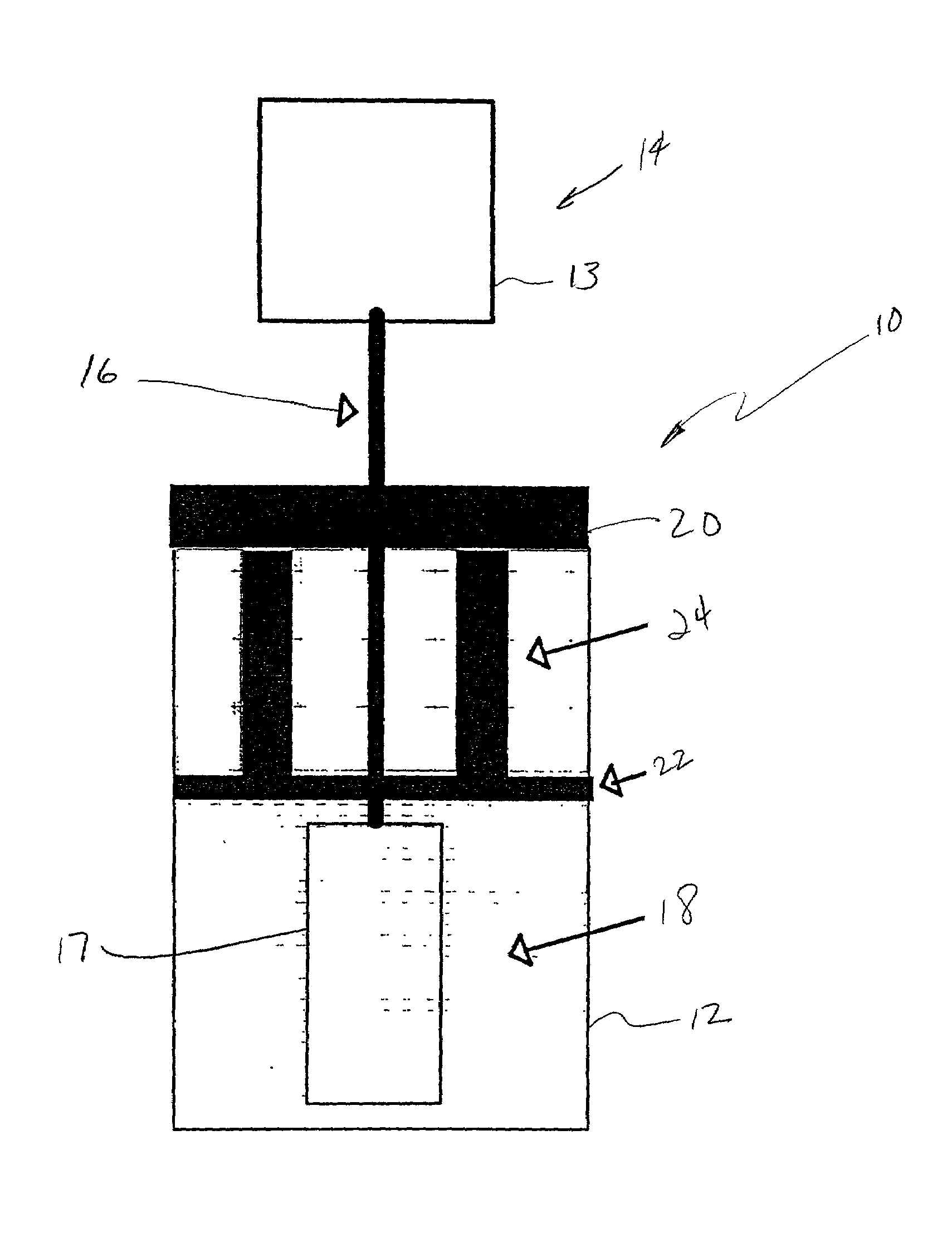
[0017]As indicated above, graft materials produced by the method of the present invention may be used for surgical procedures such as slings for treatment for urinary incontinence, surgical meshes for repair of hernias, bulking agents for cosmetic or reconstructive surgery, abdominal wall reconstructions, pelvic floor reconstructions, heart valve replacements, pericardium repairs, or arterial transplants.
[0018]While the method of the present invention may be utilized to produce immunilogical inert graft material from tissues such as the arteries, heart valves, bone, or other organs and tissues of h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| immunologically inert | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com