Method of detecting and evaluating angiotensinogen receptor-modulating compounds using placental cells
a technology of angiotensinogen receptor and placental cells, which is applied in the field of detecting and evaluating angiotensinogen receptormodulating compounds using placental cells, can solve the problems of inability to definitively answer the question whether agt is produced by placental tissue, the production of agt protein itself, and the identification of a renin-angiotensinogen receptor in native human placental cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
AGT Binding
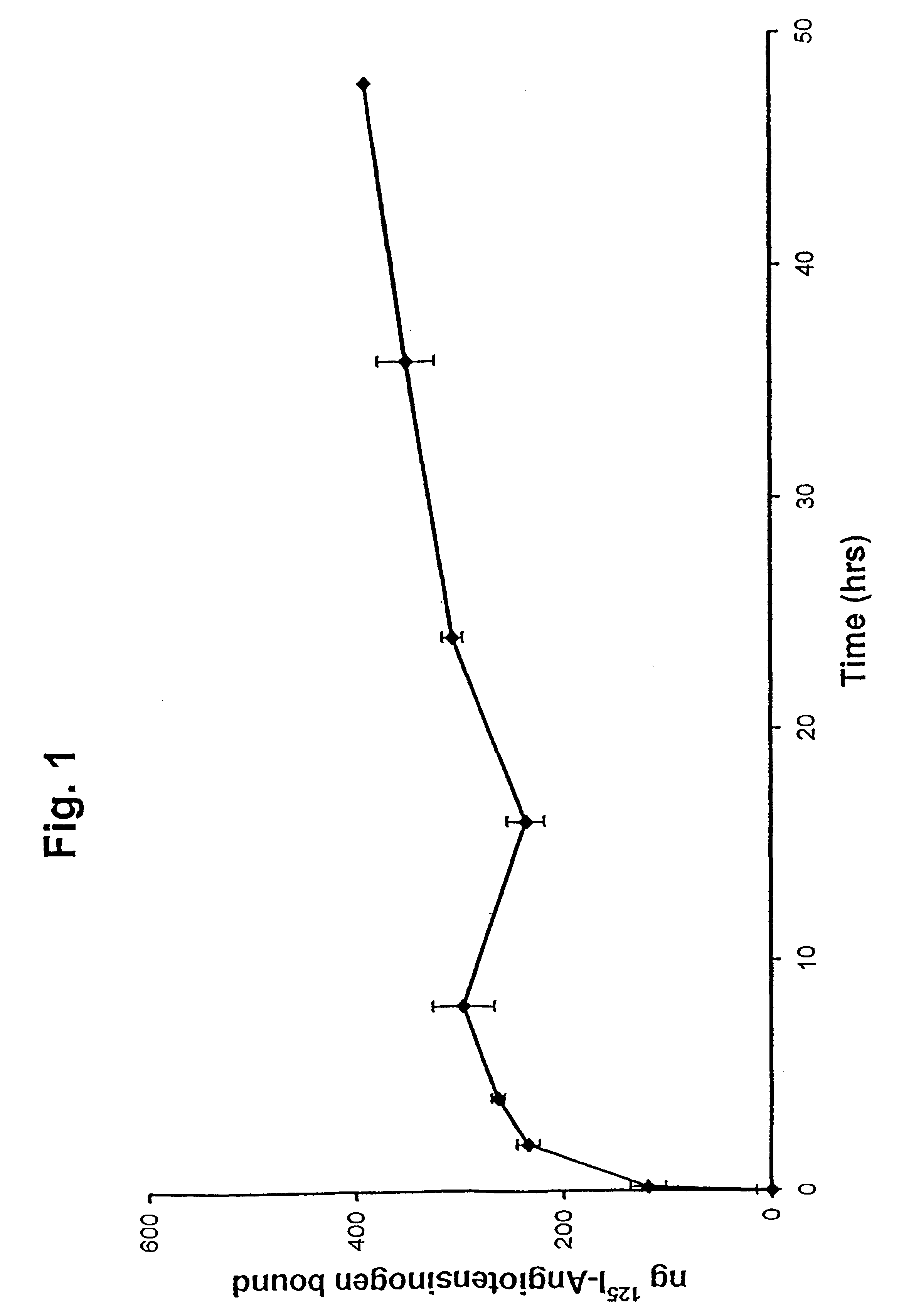
[0035]Labeled AGT, 3 μg, was added to each well containing 1 ml DEME and 2-3×104 cells. The cells were harvested at 5 min, and 2, 4, 8, 16 and 24 h, washed five times with DEME, lysed in a buffer containing 0.1 mol / L Tris, 2 mmol / L EDTA, 1% Triton-X 100, 1 μmol / L DTT, pH 7.8, and the radioactivity of the whole lysate measured. Each experiment contained two duplicates and the reported values are the mean of two experiments. The results are presented graphically in FIG. 1
example 2
Competitive Binding Study
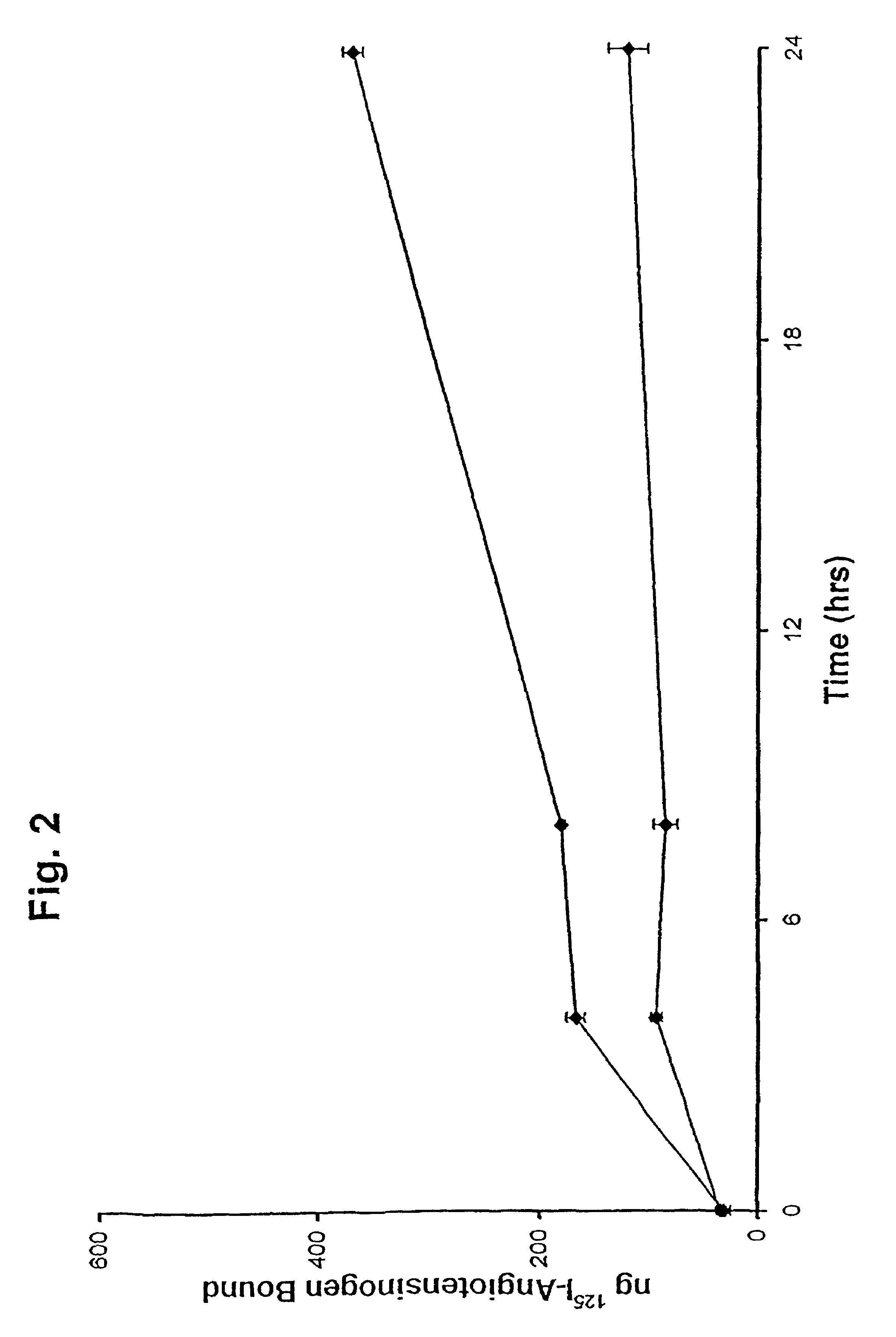
[0036]This Example was carried out as described in Example 1, except that a 100-fold excess of unlabeled AGT was added at zero time. The cells were harvested at 5 min, and 4, 8 and 24 h. The results are presented graphically in FIG. 2
example 3
Competitive Displacement Study
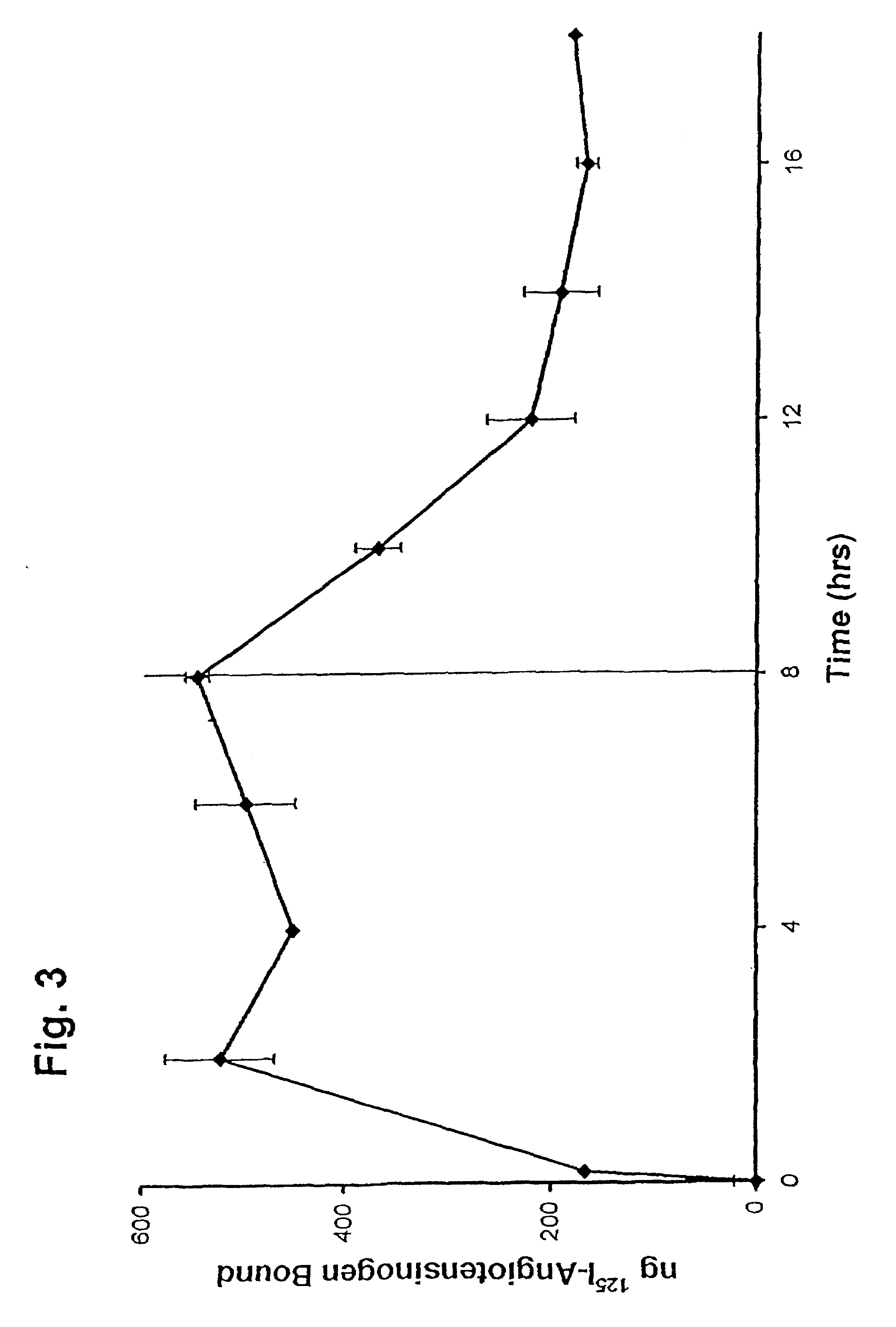
[0037]This Example was carried out as described in Example 1, except that at 8 h, a 200-fold excess of unlabeled AGT was added to duplicate wells. The cells were harvested at 5 min and at 2 intervals through 18 h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com