Combination therapy
a technology of phosphatidylinositol 3 and combination therapy, which is applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve problems such as refractory to treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of COMPOUND A
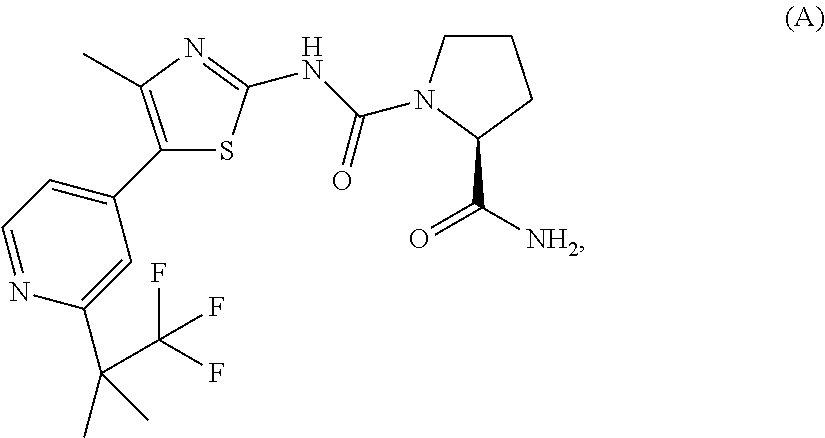
[0122]The synthesis of COMPOUND A is described in International Patent Application WO2010 / 029082, which is incorporated by reference in its entirety. The synthesis of this compound is described below.
(S)-Pyrrolidine-1,2-dicarboxylic acid 2-amide 1-{[5-(2-tert-butyl-pyridin-4-yl)-4-methyl-thiazol-2-yl]-amide}
[0123]
[0124]Et3N (1.54 mL, 11.1 mmol, 3 eq) is added to a solution of imidazole-1-carboxylic acid [5-(2-tert-butyl-pyridin-4-yl)-4-methyl-thiazol-2-yl]-amide (Step 1.1) (1.26 g, 3.7 mmol) and L-prolinamide (0.548 g, 4.8 mmol, 1.3 eq) in DMF (25 mL), under an argon atmosphere. The reaction mixture is stirred for 14 h at rt, quenched by addition of a saturated solution of NaHCO3, and extracted with EtOAc. The organic phase is washed with a saturated solution of NaHCO3, dried (Na2SO4), filtered and concentrated. The residue is purified by silica gel column chromatography (DCM / MeOH, 1:0→94:6), followed by trituration in Et2O to afford 1.22 g of the title compou...
example 2
Phase Ib / II Open-Label, Multi-Center Study of the Combination of Alpha-Isoform Specific PI3K Inhibitor COMPOUND A and Insulin-Like Growth Factor-1 Receptor (IGF-1R) Inhibitor ANTIBODY A in Adult Patients with Selected Advanced Solid Tumors
[0143]A multi-center, open-label, phase Ib / II study is conducted evaluating the efficacy and safety of the combination of the alpha-isoform specific PI3K inhibitor COMPOUND A and the Insulin-like growth factor-1 receptor (IGF-1R) inhibitor ANTIBODY A in adult patients with selected advanced solid tumors. First, a dose-escalation Phase Ib study is conducted to estimate the maximal terminal dose(s) (MTDs) and / or to identify the recommended Phase II dose(s) (RP2D) for the combination of the alpha-isoform specific PI3K inhibitor COMPOUND A and Insulin-like growth factor-1 receptor (IGF-1R) inhibitor ANTIBODY A in patients with PIK3CA mutated or amplified solid tumors. Second, a Phase II study is conducted to assess the antitumor activity and safety of ...
example 3
Targeting PIK3CA Mutant Breast Cancer with the Combination of Alpha-Isoform Specific PI3K Inhibitor, COMPOUND A, and IGF1-R Antibody, ANTIBODY A
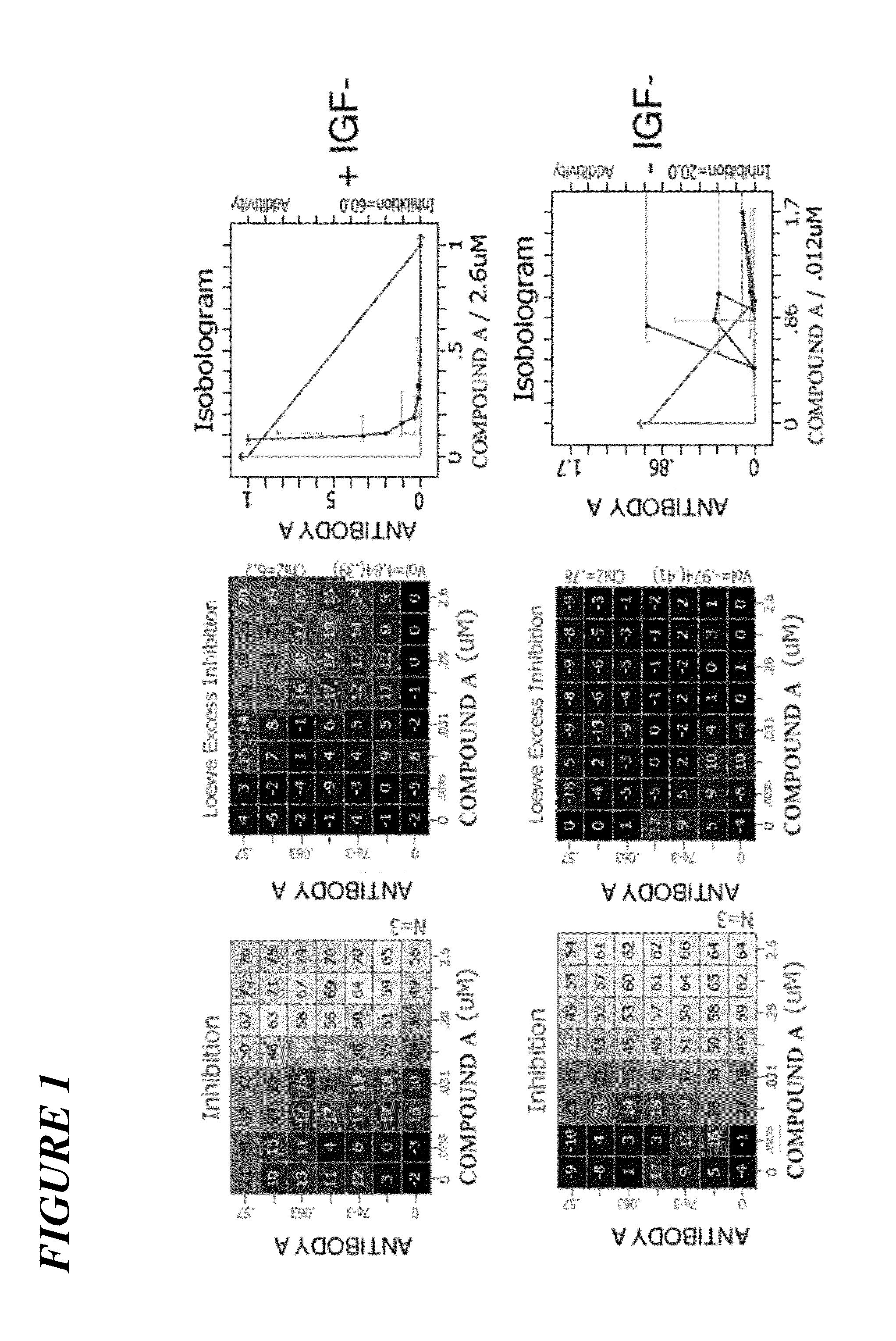
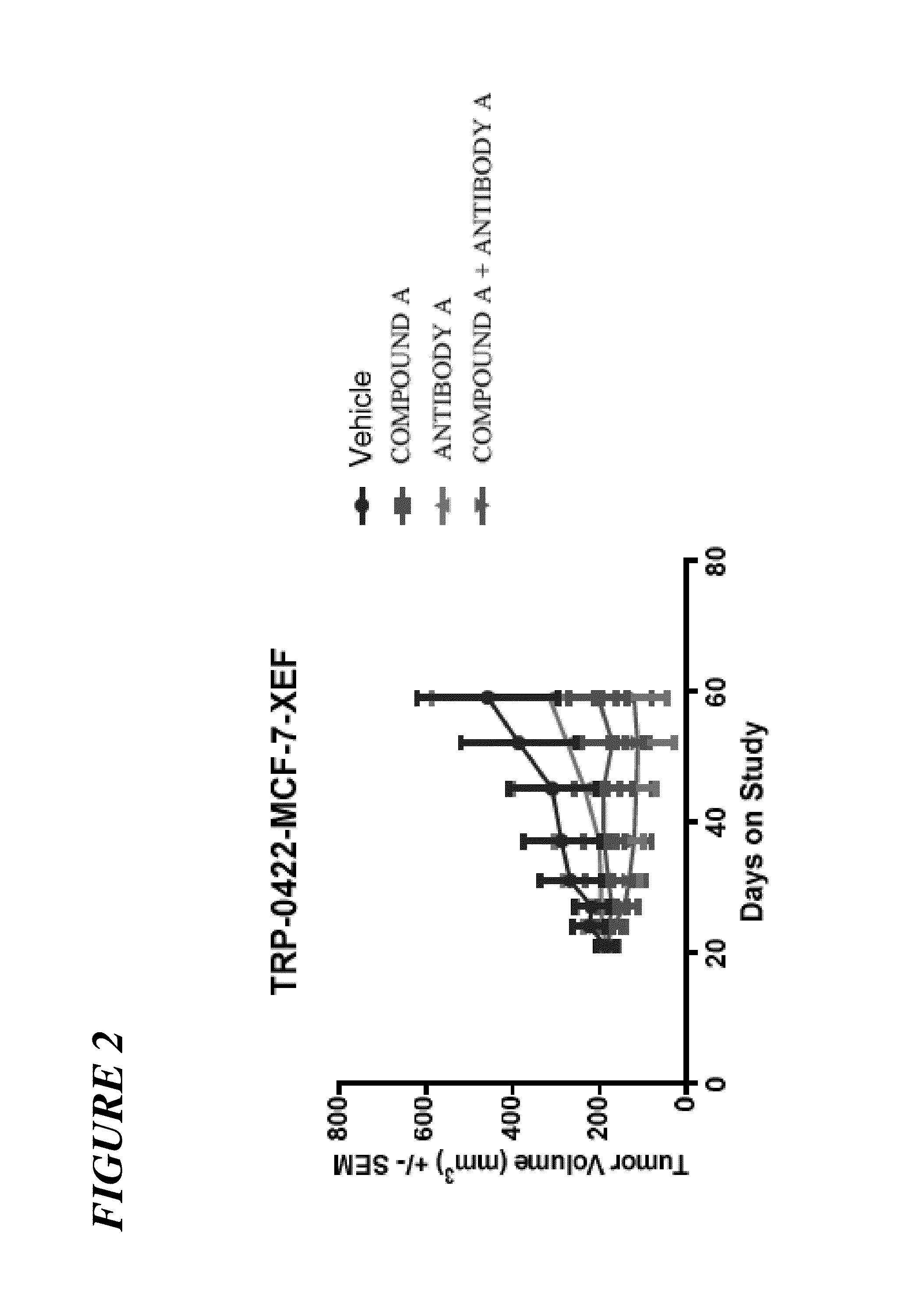
[0229]The experiments below examined whether PIK3CA inhibition would also trigger IGF1-R / IRS signaling. In addition, the combination of alpha-isoform specific PI3K inhibitor, COMPOUND A, and a fully human antibody against IGF1-R, ANTIBODY A, preclinically against a PIK3CA mutant breast cancer model, MCF7, was explored. The luminal breast cancer cell line MCF7 carries an activitating PIK3CA somatic mutation. The data indicate that IGF1-R / IRS signaling is activated upon PIK3CA inhibition. COMPOUND A exhibited concentration-dependent tumor growth inhibition in vitro. ANTIBODY A alone had modest inhibitory activity. The combination of COMPOUND A and ANTIBODY A inhibited MCF7 growth synergistically in vitro (Experiment 1). This combination was further tested in an MCF7 xenograft in mice (Experiment 2). COMPOUND A monotherapy resulted in tumor sta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com