Process for manufacturing a stationary state of crystalline polymer of a biodegradable polymer matrix carrying an active substance and a polymer matrix produced thereby
a biodegradable polymer matrix and stationary state technology, applied in the field of manufacturing, can solve problems such as thermal degradation of the polymer matrix, and achieve the effect of improving the storage stability of the polylactide matrix
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
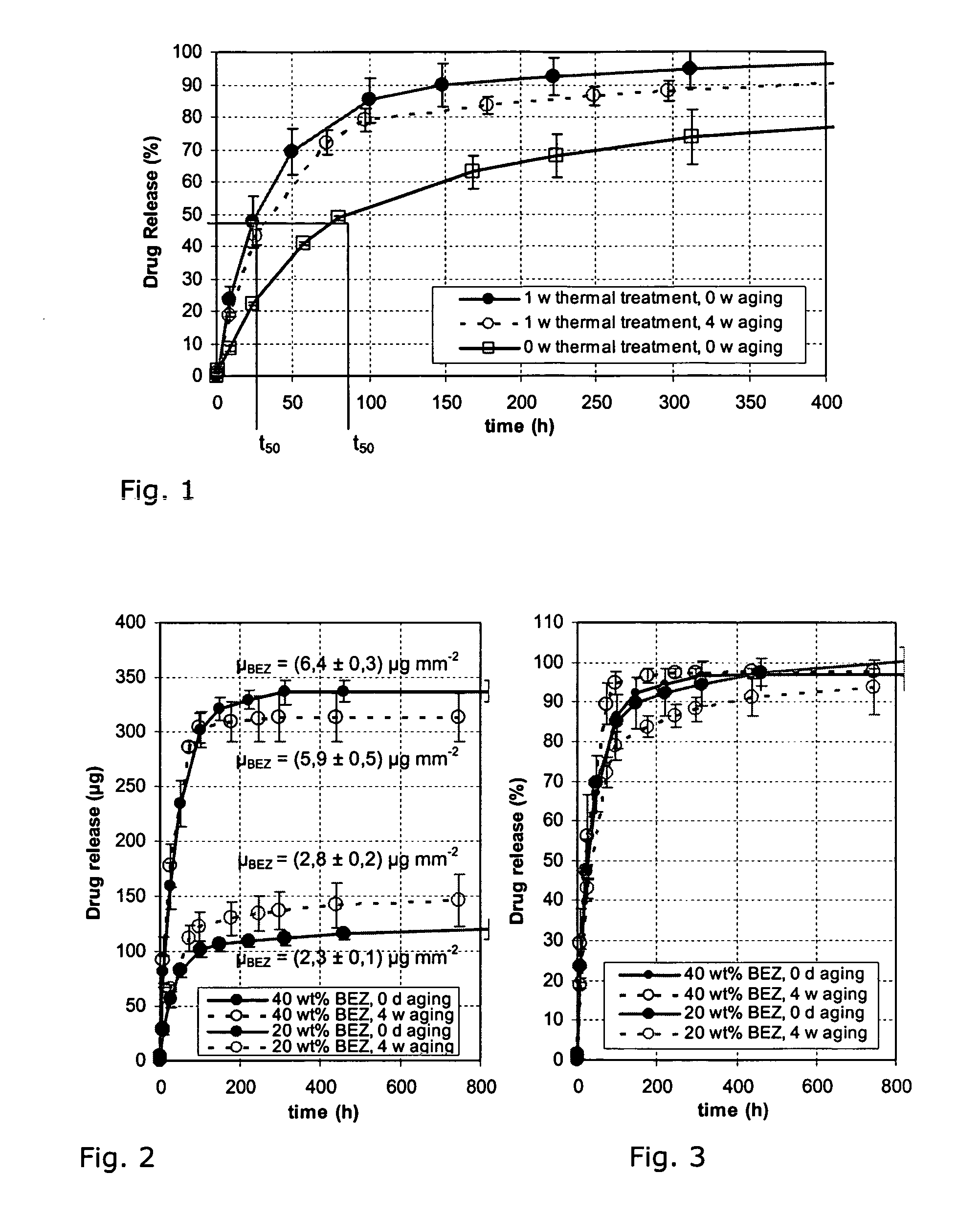
[0026]A stent of medical steel was coated by means of a rotary atomiser as follows:
[0027]The material properties for poly-L-lactide are known from the relevant literature and shown in Table 1:
[0028]
TABLE 1PropertyPoly-L-lactideProportions D, L / L[mol %]100 / 0Degree of crystallinity □[%]Glass transition temperature TG[° C.]60-70Melting temperature TS[° C.]170-180Molecular weight Mw[g / mol]SolventCHCl3, CH3ClDensity □amorph / □crystalline[g / cm3]1.25-1.29 / ~1.45Modulus of elasticity EI)[N / mm2]Tensile strength □δ1)[N / mm2]Bending strength □b1)[N / mm2]Elongation at rupture □1)[%]
[0029]The poly-L-lactides used, from Boehringer-Ingelheim, Germany, have the specific material properties indicated in Table 2:
[0030]
TABLE 2SpecificationL210L214Composition L / D, L[mol %]100 / 0 SolubilityChloroform,methyl chlorideResidual content of monomer / solvent[%] ≦0.1 / ≦0.089Metal residues tin / other[ppm]≦100 / ≦10 Water content / sulphur content[%]≦0.5 / ≦0.1Inherent viscosity[dl / g]2.8 8.0 Molecular weight Mw (GPC / MHE)[kg mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com