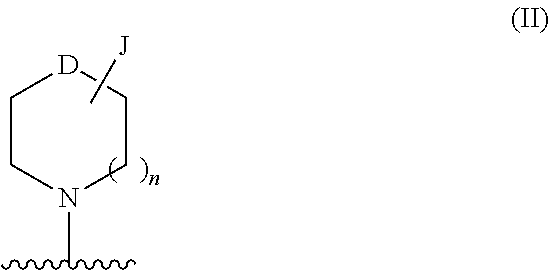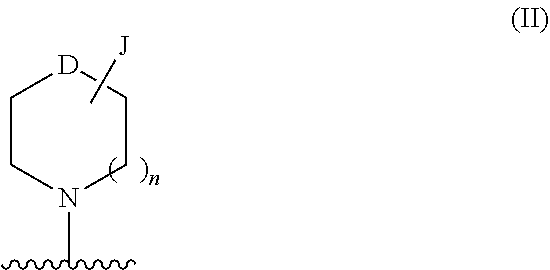Thieno-pyrimidines, useful as potassium channel inhibitors
a potassium channel inhibitor and thienopyrimidine technology, applied in the field of compounds, can solve the problems of side effects precluding its long-term use, ischemic insults,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
specific embodiments
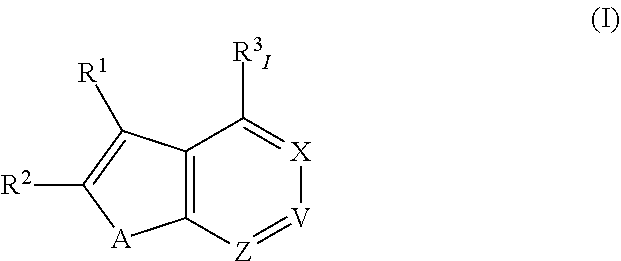
[0185]In one embodiment:
[0186]A is S;
[0187]X is N;
[0188]V is CR3III;
[0189]Z is N;
[0190]R1 is selected from optionally substituted alkyl, optionally substituted cycloalkyl, optionally substituted aryl, and optionally substituted heteroaryl;
[0191]R2 is selected from H, halo, —CN, trifluoromethyl, optionally substituted alkyl, optionally substituted alkoxy, —NR4R5, —NR6C(O)R7, —NR6S(O)2R7, —S(O)2NR4R5, —CONR4R5, —CO2R7, optionally substituted oxazolinyl, —SR14, —S(O)R14 and —S(O)2R14;
[0192]R3I is selected from H, halo, —CN, trifluoromethyl, optionally substituted alkyl, optionally substituted alkoxy, optionally substituted heterocycloalkoxy, —NR6C(O)R7, —NR6S(O)2R7, —S(O)2NR4R5, —CONR4R5, —CO2R7, —NR8R9, —C≡C-J, optionally substituted cycloalkyl-J and —(NRaRb)-J;
[0193]R3III is selected from H, halo, —CN, trifluoromethyl, optionally substituted alkyl, optionally substituted alkoxy, optionally substituted heterocycloalkoxy, optionally substituted heterocycloalkylalkyl, —NR6C(O)R7, —NR6S(...
example 58
[1169]4-[4-(Bromomethyl)-1-piperidyl]-5-phenyl-thieno[2,3-d]pyrimidine (13.8 g, 35.5 mmol), 2-(1-methylpyrrolidin-2-yl)ethanamine (5.47 g, 42.6 mmol) and potassium carbonate (7.37 g, 53.3 mmol) were heated in acetonitrile (100 mL) in the microwave at 150° C. for 30 minutes in 8×20 mL microwave vials. The reaction mixtures were combined and diluted with DCM (250 mL). The organic was washed with water (250 mL) and concentrated at reduced pressure. The resulting residue was purified by flash chromatography, eluting with a gradient of DCM to 90:10:1 DCM / MeOH / NH4OH to afford the target compound (4.0 g) and mixed fractions. LCMS [M+H]+=436.0
[1170]Other compounds prepared by Method A as described for example (iii) using the appropriate starting materials are listed in TABLE 1.
Method B
Synthesis of 1-[4-[4-[[methyl-(1-methylpyrrolidin-3-yl)amino]methyl]-1-piperidyl]-5-phenyl-thieno[2,3-d]pyrimidin-2-yl]pyrrolidine-3-carboxamide
Example 120
[1171]i) [1-(2-chloro-5-phenyl-thieno[2,3-d]pyrimidin-...
example 120
[1189]N—[[1-(2-chloro-5-phenyl-thieno[2,3-d]pyrimidin-4-yl)-4-piperidyl]methyl]-N,1-dimethyl-pyrrolidin-3-amine (100 mg, 0.219 mmol) and pyrrolidine-3-carboxamide (300 mg, 2.63 mmol) were stirred in dry acetonitrile (1.5 ml) and heated in a microwave at 140° C. for 0.5 h. The solvent was evaporated and the crude residue purified by flash column chromatography (0 to 5% 7.0M NH3 / MeOH in dichloromethane) to give 1-[4-[4-[[methyl-(1-methylpyrrolidin-3-yl)amino]methyl]-1-piperidyl]-5-phenyl-thieno[2,3-d]pyrimidin-2-yl]pyrrolidine-3-carboxamide as an off white solid (25 mg, 21%). m / z (M+H)+534.3, RT=2.69 min.
[1190]Other compounds prepared by Method B as described for example (iv) using the appropriate starting materials are listed in TABLE 1.
Method C
Synthesis of N,N-dimethyl-5-phenyl-4-[4-(2-pyrrolidin-1-ylethoxymethyl)cyclohexyl]thieno[2,3-d]pyrimidine-6-carboxamide
Example 135
[1191]i) Methyl 5-phenyl-4-[4-(2-pyrrolidin-1-ylethoxymethyl)-1-piperidyl]thieno[2,3-d]pyrimidine-6-carboxylate
[1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com