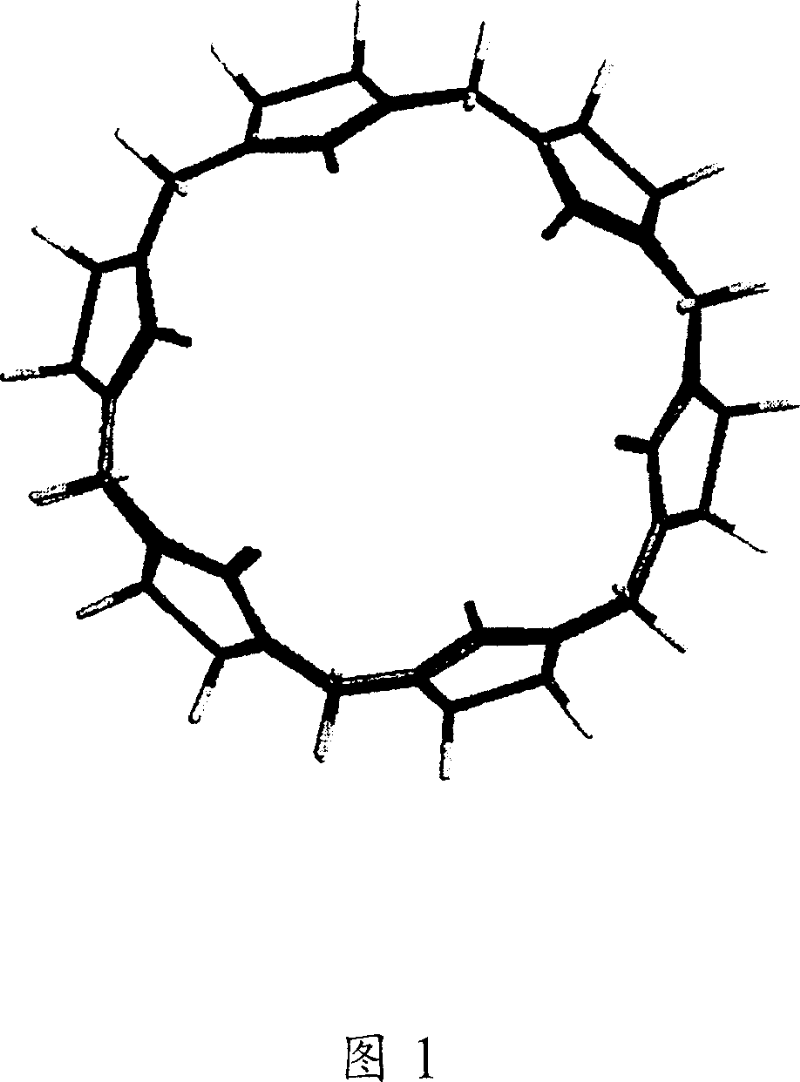
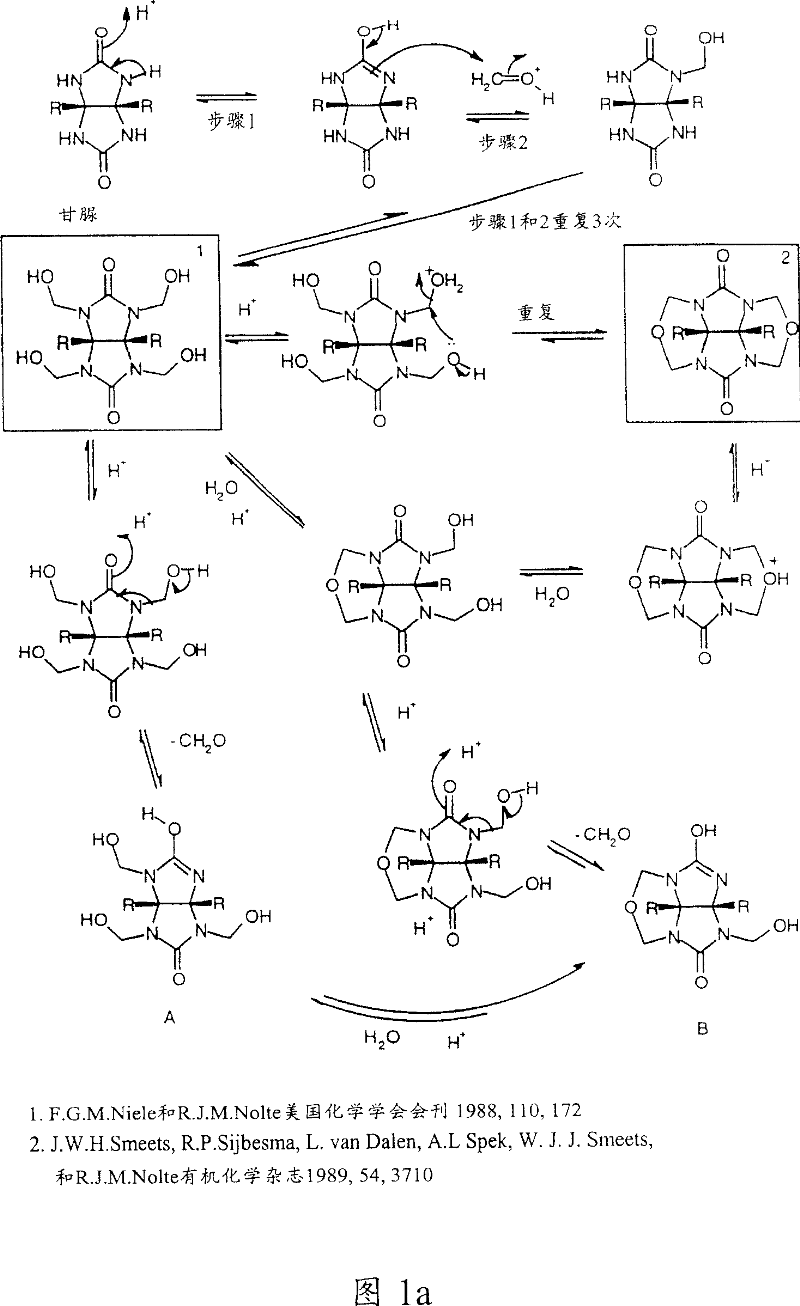
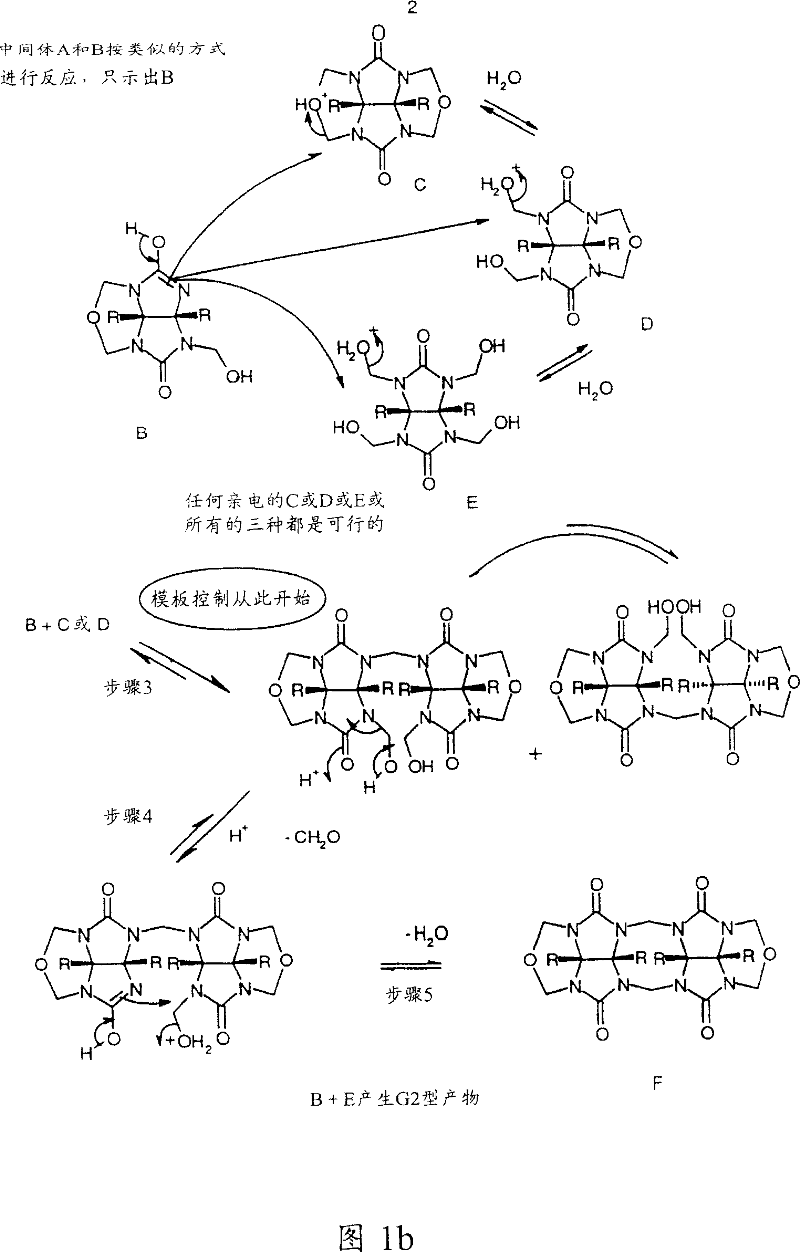
Cucurbiturils and method for synthesis
A cucurbit-shaped, glycoluril technology, used in organic chemistry and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Synthesis of Cucurbituride[n]
[0079] 1.5g-glycoluril
[0080] 6.9ml - Inorganic acid (36% hydrochloric acid, 48% hydrobromic acid, 47% hydroiodic acid or 98% or 50% sulfuric acid) or organic acid (p-toluenesulfonic acid)
[0081] 1.5ml-30% aqueous formaldehyde
[0082] 5 mmol - the corresponding alkali metal halide, ammonium halide or in the case of sulfuric acid the corresponding sulfate or alkali metal tosylate.
[0083] 600 mg - red phosphorus (this red phosphorus is added to the reaction mixture when using hydriodic acid)
[0084] Glycoluril (1.5 gm, 10.6 mmol) was dissolved or suspended in the appropriate acid (6.9 ml). The alkali metal or ammonium salt (5 mmol) of the corresponding anion belonging to the acid is then added in the case of salts to control the reaction product. Formaldehyde (1.5 ml) was added to the mixture at room temperature over a period of 5-10 minutes, and the mixture coagulated into a gel (Note 1). After standing still for 3 hours (Note...
Embodiment 2
[0101] Synthesis of Cucurbituride [s, u]
[0102] The same pattern control was applied to the substituted cucurbituril[n] by the method described above in which the glycoluril used was a substituted glycoluril or the method described below.
[0103] A mixture of tetracyclic ether B (2.5 mol) and glycoluril (0.355 gm, 2.5 mmol) was dissolved or suspended in the appropriate acid (6.9 ml) (Note 1). The alkali metal or ammonium salt (5 mmol) of the corresponding anion belonging to the acid is then added in the case of salts to control the reaction product. The reaction mixture was heated and kept at 100°C for 3 hours (Note 2). The reaction mixture was cooled to room temperature, the product was isolated by the addition of methanol (10 ml), and the resulting precipitate was collected by filtration. The solid material was washed with methanol and acetone and air dried. Further purification is carried out by recrystallization from aqueous hydrochloric or hydrobromic acid, or by di...
Embodiment 3
[0109] Analysis of Cucurbituril Mixture
[0110] The analysis of cucurbit uril reaction mixture was routinely used 13 C NMR method. The present invention has been able to obtain the X-ray crystal structures of cucurbit biurea [5], cucurbit biurea [8] and cucurbit biurea [10]. These structures are shown in Figure 9a, where formula 12 is cucurbit biurea [5], formula 13 is cucurbit biurea [8], and formula 14 is cucurbit biurea [10]. Crystallized water and salt etc. are not shown.
[0111] Cucurbituril
[n]
n =
Methine C
Observations *
(ppm)
Methine C
Calculated
(ppm)
Observations *
(ppm)
Calculated
(ppm)
4
-
68.54
-
48.75
5
69.84
69.87
50.58
50.68
6
70.98
70.96
52.29
52.17
7
71.90
71.88
53.48
53.43
8
72.70
72.68
54.49 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com