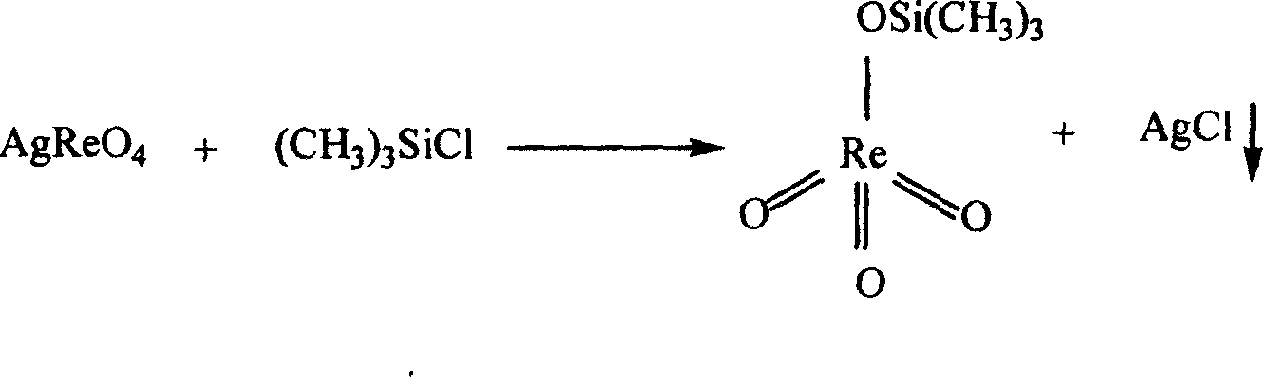
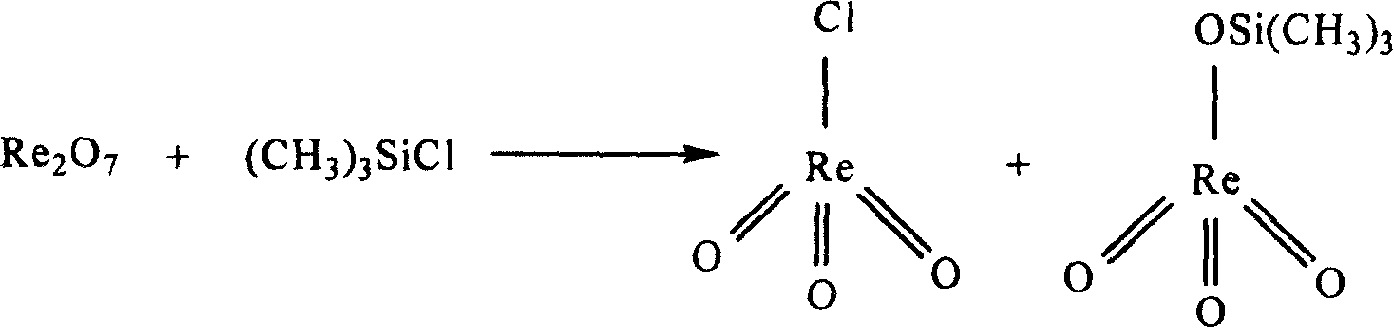
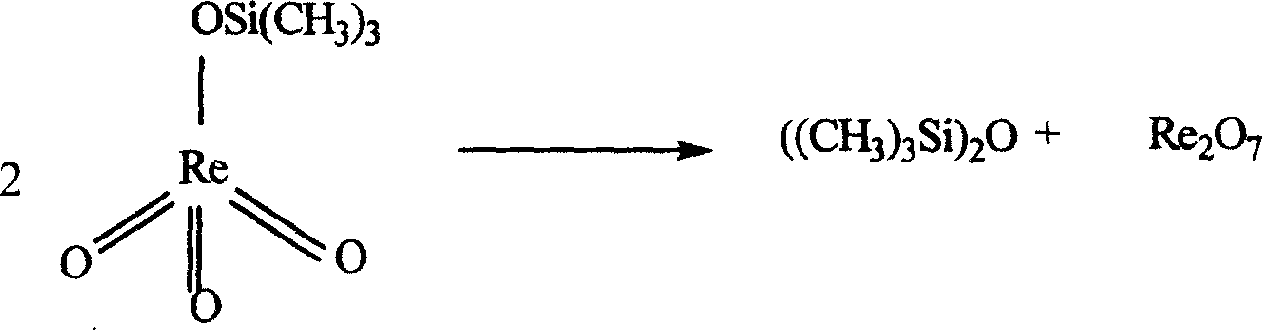Method for synthesizing methyl rhenium trioxide from perrhenate
A technology of methyl rhenium trioxide and methyl rhenium methoxide, which is applied in the field of organometallic compound manufacturing, can solve the problems of unsuitability for mass production, difficult reaction control, and harsh conditions, and achieve shortening of refining time, easy operation, and time-saving short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 1. Oxidation into salt:
[0047] Add 94g (0.5mol) rhenium powder in the reactor, add 248mL distilled water, add dropwise under stirring at room temperature 751g (11.9mol) of pre-prepared nitric acid with a mass fraction of 45%. Stir and react for 2 hours until the rhenium powder is completely dissolved, then add the silver nitrate solution dissolved in 100ml water with 85g (0.5mol) silver nitrate in advance, white precipitate will be produced in the reaction solution immediately, continue to react for 10 minutes, drop to room temperature, pump After filtration, the filter cake was washed three times with 600 mL of ether, then placed in a drying oven, and dried at 65° C. for 4 hours to obtain 171.74 g of high-purity silver perrhenate with a yield of 95%.
[0048] 2. MTO synthesis: In the reactor, under the protection of nitrogen, add 1800 mL of acetonitrile and 171.74 g (0.5 mol) of silver perrhenate at room temperature, stir for 4 to 5 minutes, and add 134 mL of pre-wei...
Embodiment 2
[0061] 1. Oxidation into salt:
[0062] Add 93.05g (0.5mol) of rhenium powder in the reactor, add 248mL of distilled water, add dropwise 740g (11.7mol) of nitric acid with a mass fraction of 45% prepared in advance under stirring at room temperature. Continue to stir and react for 2 hours, until the rhenium powder is completely dissolved, then add the potassium nitrate solution dissolved in 180ml water with 52g (0.5mol) potassium nitrate in advance, white precipitate will be produced immediately in the reaction solution, continue to react for 10 minutes, be down to room temperature, Suction filtration, the filter cake was washed three times with 600mL n-butanol, then placed in a vacuum drying oven, and vacuum-dried at 75°C at 0.1mm-Hg for 4 hours to obtain 135.90g of high-purity potassium perrhenate with a yield of 94% .
[0063] 2. Synthesis of MTO: In the reactor, under the protection of nitrogen, add 1000 mL of dichloromethane and 135.90 g (0.5 mol) of potassium perrhenate...
Embodiment 3
[0070] 1. Oxidation into salt:
[0071] Add 93.05g (0.5mol) rhenium powder in the reactor, add 248mL distilled water, add dropwise under stirring at room temperature 555g (11.7mol) of pre-prepared nitric acid with a mass fraction of 60%. Continue to stir, react 2 hours, until all dissolving of rhenium powder, then add the potassium chloride solution that is dissolved in 120ml water with 38g (0.5mol) potassium chloride in advance, produce white precipitate immediately in the reaction solution, continue to react 10 minutes, drop to Suction filtration at room temperature, the filter cake was washed three times with 600mL tetrahydrofuran, then placed in a vacuum drying oven, and vacuum-dried at 75°C 0.1mm-Hg for 4 hours to obtain 134.45g of high-purity potassium perrhenate with a yield of 93% .
[0072] 2. Synthesis of MTO: In the reactor, under the protection of nitrogen, add 1000 mL of acetonitrile and 134.45 g (0.5 mol) of potassium perrhenate at room temperature, stir for 4 t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com