Method for synthesizing series single-dispersed ferrite nanometer magnetic beads
A nano-magnetic bead, monodisperse technology, applied in the field of synthesis of ferrite nano-magnetic beads, can solve problems such as insufficient dispersion and uneven product size distribution, and achieve large performance control space, uniform product morphology, and good performance. good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Take 16mmol of FeCl3 , added to a 50 ml hydrothermal kettle, 40 ml of ethylene glycol solution was added to the kettle, after dissolving, heated at 200°C for 72 hours, the obtained black precipitate was washed with deionized water, dried at 40-80°C, and prepared Ferric oxide nano-magnetic beads with a particle diameter of 100-1000 nanometers are obtained.
[0037] Reduce FeCl 3 The usage amount is up to 2mmol, and a similar product is prepared through the same process;
[0038] Ferric sulfate, ferric nitrate, and ferric acetate are used to replace ferric chloride, and similar products are obtained through the same process.
Embodiment 2
[0040] Take 8mmol of manganese chloride and 16mmol of FeCl 3 Added to 50 ml of water heating kettle, at this time Mn 2+ with Fe 3+ The molar ratio is 1:2. Add 40 ml of ethylene glycol solution to the kettle, stir and heat at 250°C for 18 hours, wash the obtained precipitate with deionized water, and dry it at 40-80°C to obtain MnFe 2 o 4 nano magnetic beads.
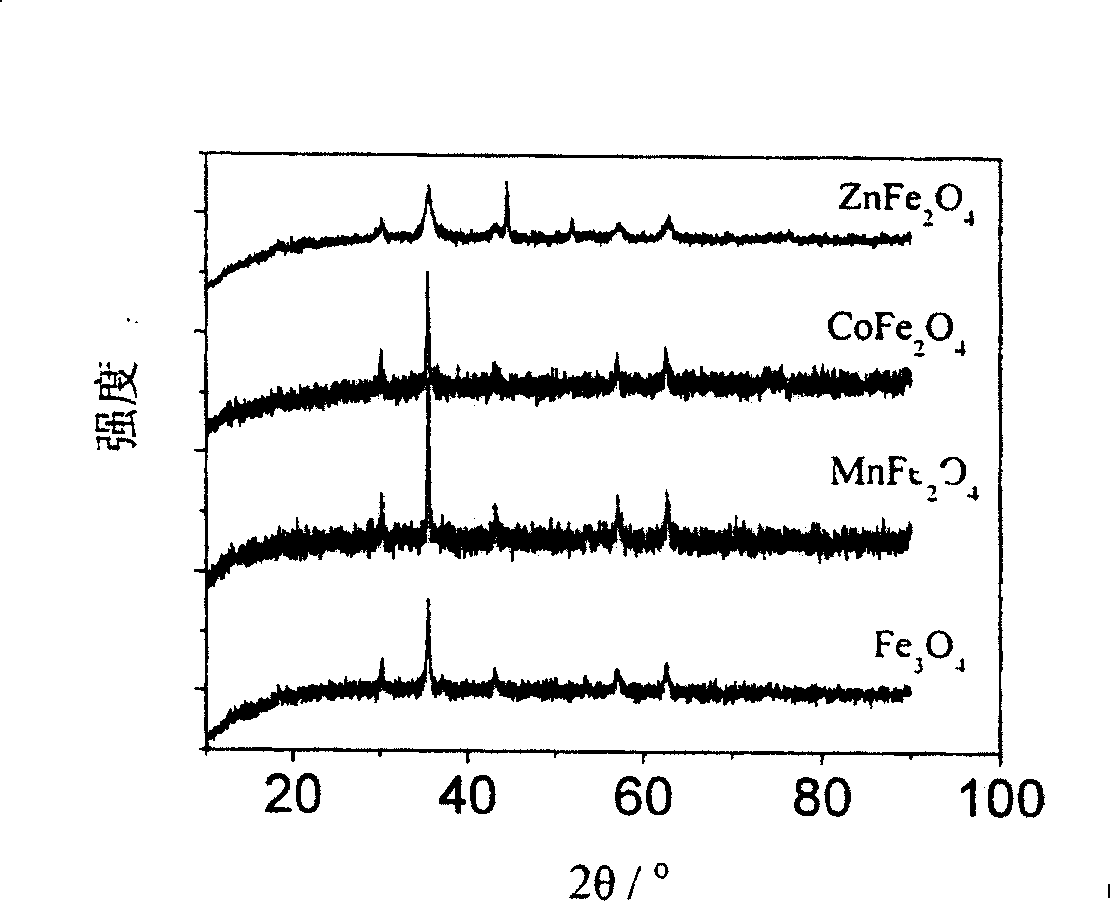
[0041] Control the molar ratio of divalent metal ions to iron ions to be 1:2, the same process is applicable to Ni 2+ , Cu 2+ , Zn 2+ , Cd 2+ , Pb 2+ , Sn 2+ , Ca 2+ ,Sr 2+ , Ba 2+ , Cd 2+ , Mg 2+ ,Co 2+ The reaction of soluble salts of divalent metal ions and soluble ferric ion salts, finally forming NiFe 2 o 4 , CuFe 2 o 4 , ZnFe 2 o 4 , CdFe 2 o 4 , PbFe 2 o 4 , SnFe 2 o 4 , CaFe 2 o 4 , SrFe 2 o 4 , BaFe 2 o 4 , CdFe 2 o 4 , MgFe 2 o 4 , CoFe 2 o 4 and other ferrite nano-magnetic beads.
Embodiment 3
[0043] Weigh 0.08mmolMnCl 2 and 16mmol of FeCl 3 , added to a 50 ml water heating kettle, at this time Mn 2+ with Fe 3 + of
[0044] The molar ratio is 0.01:2, add 40 ml of ethylene glycol solution to the kettle, heat at 300°C for 8 hours, wash the obtained precipitate with deionized water, and dry it at 40-80°C to obtain Mn x Fe 3-x o 4 Manganese ferrite compound nano magnetic beads.
[0045] control Mn 2+ The molar ratio to iron ions is x:2, where 0.01
[0046] Control the molar ratio of divalent metal ions to iron ions to be x: 2, where 0.12+ , Cu 2+ , Zn 2+ , Cd 2+ , Pb 2+ , Sn 2+ , Ca 2+ ,Sr 2+ , Ba 2+ , Cd 2+ , Mg 2+ ,Co 2+ The reaction of soluble salts of divalent metal ions and soluble ferric ion salts, and finally the formation of Ni x Fe 3-x o 4 , Cu x Fe 3-x o 4 , Zn x Fe 3-x o 4 , Cd x Fe 3-x o 4 , Pb x Fe 3-x o 4 , Sn x Fe 3-x o 4 , Ca x Fe 3-x o ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com