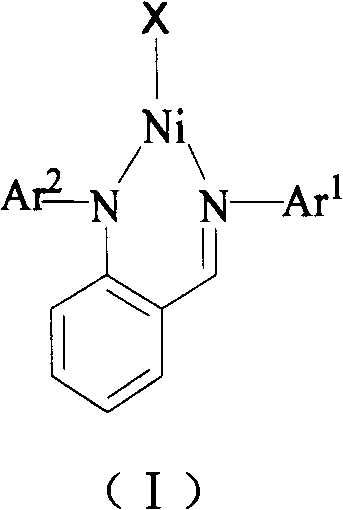
Production of irregular phenylethylene/norbornene multipolymer
A norbornene and styrene technology, applied in the chemical industry, can solve the problems of low temperature, limited polymer performance, unfavorable industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the synthesis of anilino imine nickel compound 1
[0018] Synthesis of Ligand L1:
[0019] 2-fluoro-2,6-dimethylbenzimine (2-F-C 6 h 6 (CH=NC 6 h 3 Synthesis of i-Pr-2, 6)): 11.68g 2-fluorobenzaldehyde and 10.38g 2,6-dimethylaniline were stirred in 40mL n-hexane for 2h, and MgSO was added 4 Dehydration; the obtained bright yellow solution was distilled under reduced pressure, and the fraction at 162-163° C. was collected at 0.5 cmHg to obtain 14.34 g of bright yellow viscous liquid with a yield of 73.7%. Mass Spectrum EI-MS (m / z): 228.2[M] + . Elemental Analysis C 15 h 14 FN: Theoretical: C, 79.22%; H, 6.27%; N, 6.16%. Found values: C, 79.07%; H, 6.21%; N, 6.01%.
[0020] 9.9 mL of butyl lithium (n-BuLi) n-hexane solution (2.6 mol / L) was gradually added to 2,6-dimethylaniline (3.3 mL) in tetrahydrofuran (40 mL) at -78 ° C, and the solution was stirred overnight Slowly rise to room temperature; then dissolve 5.0 mL of 2-fluoro-2,6-dimethylbenzimi...
Embodiment 2
[0023] Embodiment 2: the synthesis of anilino imine nickel compound 2
[0024] Synthesis of Ligand L2:
[0025] According to the synthesis method of ligand L1 in Example 1, 2,6-diisopropylaniline is used to replace 2,6-dimethylaniline and 2-fluoro-2,6-dimethylbenzimine to react, Other operating conditions are the same. 5.31 g of 2-fluoro-2,6-dimethylbenzimine was reacted with 5.10 mL of 2,6-diisopropylaniline and 9.9 mL of n-BuLi (2.6M) to give pale yellow crystals (ligand L2) 3.99 g, 44.4% yield. Mass Spectrum EI-MS (m / z): 385.2[M] + . Elemental Analysis C 27 h 32 N 2 : Theoretical value: C, 84.26%; H, 8.46%; N, 7.28%. Found values: C, 84.21%; H, 8.48%; N, 7.06%.
[0026] Synthesis of compound 2:
[0027] According to the same synthetic method of compound 1 in Example 1, 1.21g ligand L2 and 1.7ml n-BuLi (2.6M) and 1.35g (DME) NiBr 2 After reaction, 1.51 g of dark green solid was obtained. Yield: 66.0%. Mass Spectrum EI-MS (m / z): 521, 522, 523, 524, 525, 526, (iso...
Embodiment 3
[0028] Embodiment 3: the synthesis of anilino imine nickel compound 3
[0029] Synthesis of Ligand L3:
[0030] 2-fluoro-2,6-diisopropylbenzimine (2-F-C 6 h 6 (CH=NC 6 h 3 i PR 2 -2,6)) Synthesis: 11.5g 2-fluorobenzaldehyde and 18.1g 2,6-diisopropylaniline were stirred in 40mL n-hexane for 2h, and the resulting bright yellow solution was frozen in a refrigerator at -10°C. 13.46 g of bright yellow crystals were obtained. After the filtrate was concentrated and cooled, another 4.3 g of crystals were obtained, and a total of 17.68 g of crystals was obtained. Yield 68%. Mass Spectrum EI-MS (m / z): 284.3 [M] + . Elemental Analysis C 19 h 22 NF: Theoretical: C, 80.53%; H, 7.83%; N, 8.94%. Found: C, 80.37%; H, 7.54%; N, 4.73%.
[0031] 14.8mL of n-BuLi hexane solution (2.6M) was slowly added dropwise into a 7.65mL 2,6-diisopropylaniline solution in tetrahydrofuran (40mL) at -78°C, stirred overnight and slowly rose to room temperature; then 10 g of 2-fluoro-2,6-diisopropy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com