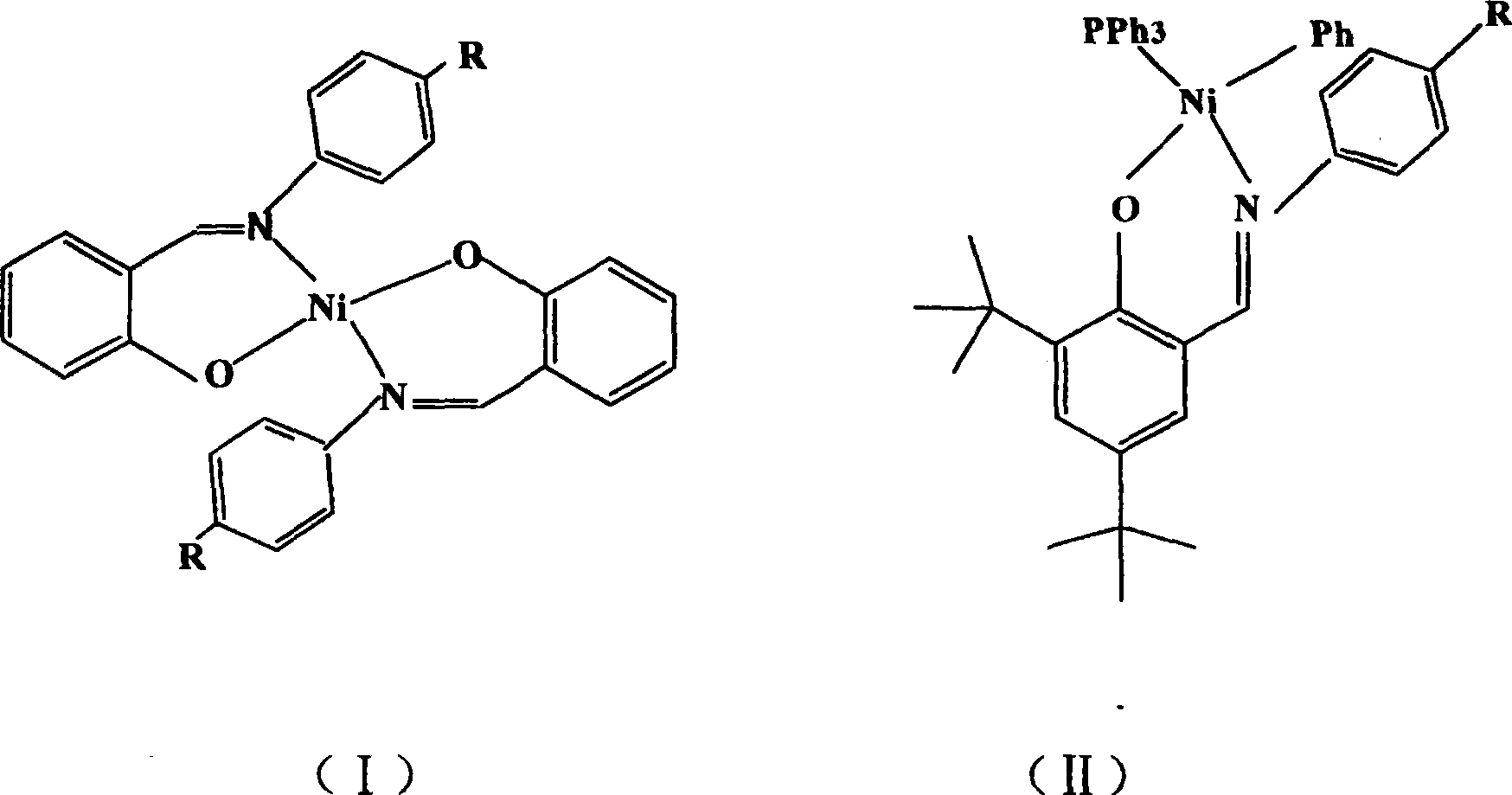
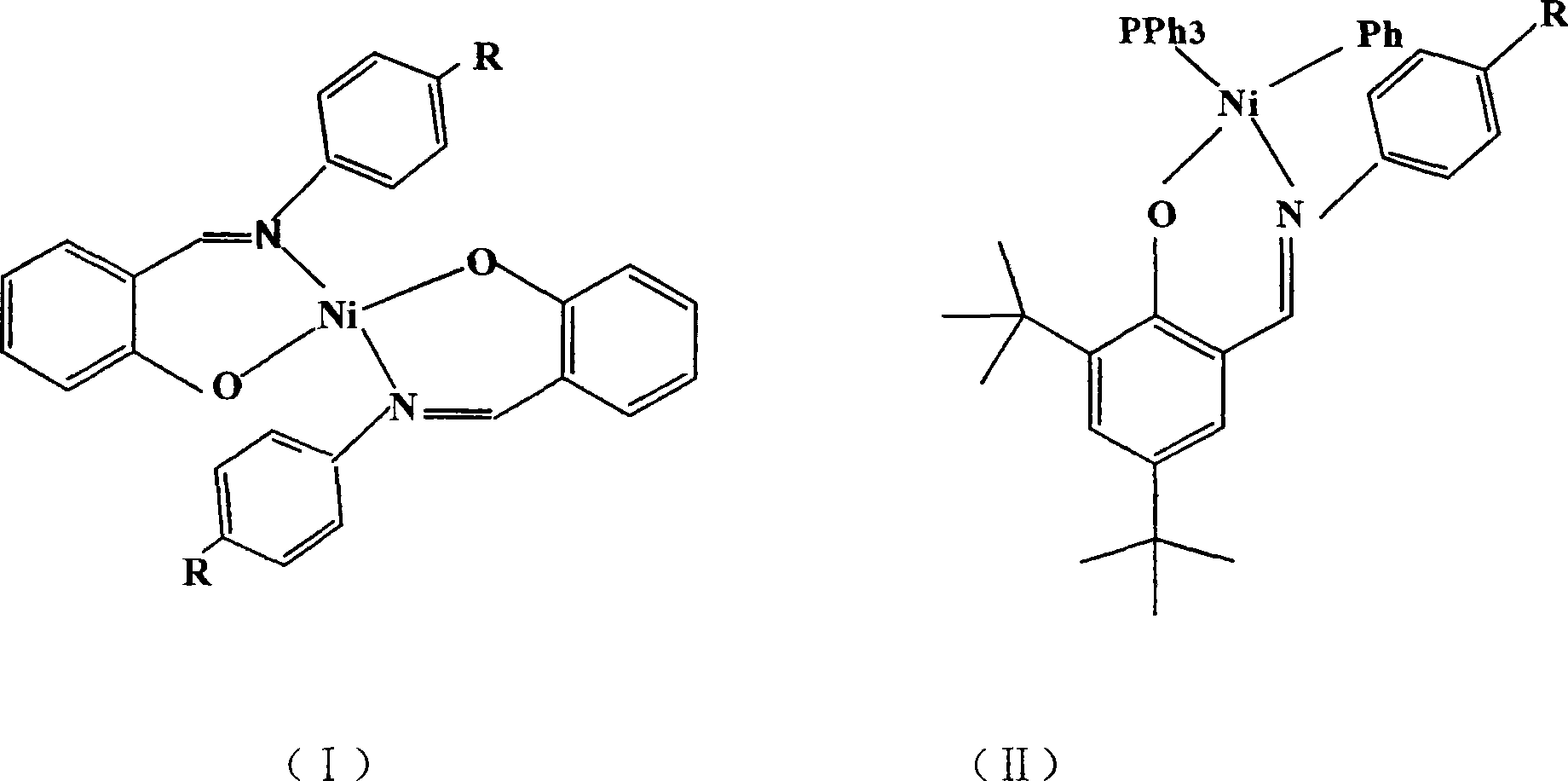
Preparation method of cyclolefin copolymer
A technology of styrene and polymerization, applied in the field of cyclic olefin copolymers, which can solve the problems of limited polymer properties, low temperature, and unfavorable industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the synthesis of salicylaldimine nickel compound 1
[0018] Ligand L 1 Synthesis:
[0019] Add 3mL (28mmol) of salicylaldehyde and 3.86g (28mmol) of p-nitroaniline to 80mL of absolute ethanol, and dropwise add 0.5mL of formic acid to reflux reaction for five hours, stop heating, and place the reaction bottle in the refrigerator overnight, with an orange-red solid Precipitated, filtered and dried to obtain 6.3 g of orange-red powder with a yield of 93%. Then recrystallize from a mixture of ethanol and tetrahydrofuran to obtain orange-red crystals. Elemental analysis measured value (%): N, 11.75; C, 64.91; H, 4.11; theoretical value (%): N, 11.55; C, 64.45; H, 4.13; 1 HNMR: (300MHZ, CDCl 3 , δ): 12.55 (s, 1H), 8.63 (s, 1H), 8.31 (d, 2H), 7.44 (m, 4H), 7.00 (m, 2H).
[0020] Synthesis of compound 1:
[0021] Vacuumize the 100mL flask with a branch pipe and fill it with nitrogen, feed nickel acetate hexahydrate, salicylaldehyde, and the corresponding p-n...
Embodiment 2
[0022] Embodiment 2: the synthesis of salicylaldimine nickel compound 2
[0023] Ligand L 2 Synthesis:
[0024] According to the ligand L in Example 1 1 3mL (28mmol) salicylaldehyde and 3.45g (28mmol) p-methoxyaniline were added to 80mL absolute ethanol, and 0.5mL formic acid was added dropwise for reflux reaction for five hours, heating was stopped, and the reaction bottle was placed in the refrigerator overnight , a yellow-green solid was precipitated, which was filtered and dried to obtain 5.8 g of a solid, with a yield of 91%. Then recrystallize with ethanol to obtain yellow-green scale-like crystals. Elemental analysis measured value (%): N, 6.11; C, 73.65; H, 5.69; theoretical value (%): N, 6.15; C, 73.91; H, 5.71; 1 HNMR: (300MHZ, CDCl 3 , δ): 13.44 (s, 1H), 8.593 (s, 1H), 7.367 (d, 2H), 7.273 (d, 2H), 6.90-7.1 (m, 4H), 3.844 (s, 3H).
[0025] Synthesis of compound 2:
[0026] The synthesis method is the same as the preparation of compound 1. Equimolar p-methoxya...
Embodiment 3
[0027] Embodiment 3: the synthesis of salicylaldimine nickel compound 3
[0028] Ligand L 3 Synthesis:
[0029] Add 125g (0.61mol) of 2,4-di-tert-butylphenol, 170g (1.21mol) of hexamethylenetetramine, and 300mL of glacial acetic acid into a 2L three-necked round-bottomed flask, stir magnetically, and within one hour Slowly raise the temperature to 130°C, keep the temperature for 2 hours, cool to 75°C and add 300mL of 33% (W / W) H 2 SO 4 Aqueous solution, under magnetic stirring, the solution is heated to 105°C and refluxed for 1 hour, the stirring and heating are stopped, and then cooled to 75°C, and the reaction mixture is transferred to a separating funnel at a constant temperature, and the organic phase is separated and transferred to 1L In the reaction bottle, after cooling to 50°C, 100 mL of methanol was added, and the mixture was cooled in a refrigerator. A light yellow solid precipitated out. After vacuum filtration, the obtained solid was recrystallized in methanol t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com