Preparation of composite drug-loaded nanoparticles of carboxyl-chitosan and chitosan modified by glycyrrhizanates
A technology of carboxylated chitosan and glycyrrhizinate, which is applied in the field of medicine, can solve problems not related to the active targeting function of drug-loaded nanoparticles, and achieve the effect of a safe and reliable treatment plan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Preparation of Glycyrrhizic Acid Monoamine Salt Modified Chitosan / Carboxylated Chitosan Composite Blank Nanoparticles
[0022] (1) Configuration of chitosan acidic aqueous solution: get chitosan (molecular weight 40k, degree of deacetylation > 90%, Zhejiang Jinke Biochemical Co., Ltd.), and configure 5mg / mL chitosan with 0.3% acetic acid solution solution, adjusted to pH 6.0 with ammonia water, and filtered for later use.
[0023] (2) Configuration of carboxylated chitosan aqueous solution: take carboxypropyl chitosan (purchased from Zhejiang Jinke Biochemical Co., Ltd.), add distilled water to configure a 4mg / mL solution, add glycyrrhizic acid monoamine salt, adjust the final The concentration is 0.3mg / mL, adjusted to pH 8.5 with ammonia water, and filtered for later use.
[0024] (3) Preparation of composite nanoparticles: Take 10mL of the solution in (2), slowly add 4mL of the solution in (1) under stirring at room temperature (rotating speed is 1000r / min...
Embodiment 2
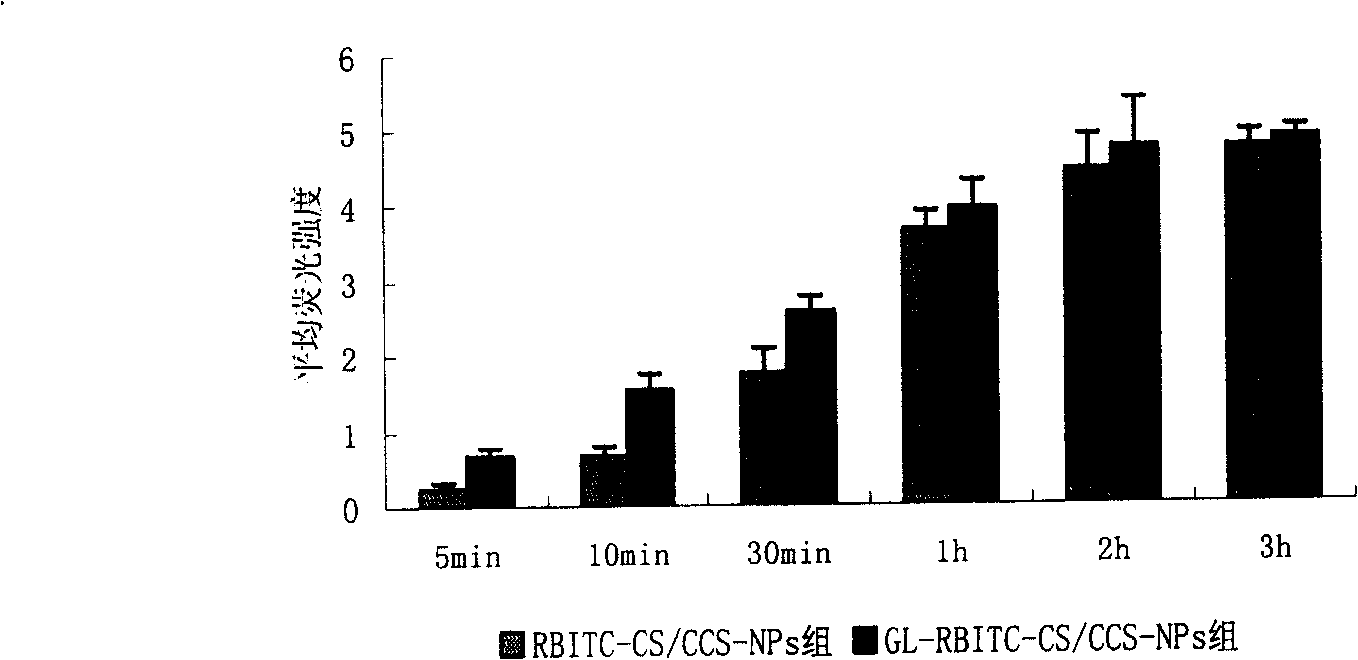
[0025] Example 2 Glycyrrhizinate Modified Chitosan / Carboxylated Chitosan Composite Blank Nanoparticles Binding Effect on Liver Parenchymal Cells
[0026](1) Fluorescent labeling of chitosan: get 100mL of 2.5% chitosan solution, adjust the pH to 8.5 with 1mol / L NaOH solution, add 1mg / mL of fluorescent dye rhodamine B isothiocyanate (rhodamine B isothiocyanate, RBITC, sigma) 100uL, reacted in the dark for 1h, free RBITC was removed by dialysis with a dialysis bag in the dark, and RBITC-labeled chitosan solution (RBITC-CS) was obtained. Take an appropriate amount and configure it to 5mg·mL -1 RBITC-CS solution.
[0027] (2) The operation steps are the same as those in Example 1 to obtain fluorescently labeled glycyrrhizic acid monoamine salt modified chitosan / carboxylated chitosan composite blank nanoparticles, constant volume 20mL (referred to as GL-RBITC-CS / CCS-NPs group) Operation steps are the same as example 1, only do not add glycyrrhizic acid monoamine salt in carboxylat...
Embodiment 3
[0030] Example 3 Preparation of Glycyrrhizic Acid Diamine Salt Modified Chitosan / Carboxylated Chitosan Composite Adriamycin Nanoparticles
[0031] (1) Configuration of chitosan acidic aqueous solution: get chitosan (molecular weight 100k, degree of deacetylation > 90%, Zhejiang Jinke Biochemical Co., Ltd.), and configure 2mg / mL chitosan with 0.5% acetic acid solution Add an appropriate amount of doxorubicin hydrochloride to the solution so that the final concentration is 4 mg / mL, adjust the pH to 6.5 with ammonia water, and filter for later use.
[0032] (2) Configuration of carboxylated chitosan aqueous solution: take carboxypropyl chitosan (Zhejiang Jinke Biochemical Co., Ltd.), add distilled water to configure a 2mg / mL solution, add diamine glycyrrhizinate, and the final concentration is 1mg / mL, adjusted to pH 8.5 with ammonia water, and filtered for later use.
[0033] (3) Preparation of composite drug-loaded nanoparticles: Take 10 mL of the solution in (2), slowly add 4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com