Inhibition peptide in domain of polymerase protein of hepatitis b virus, and application
A technology of hepatitis B virus and polymerase protein, which is applied in the fields of molecular biology and biochemistry of biotechnology, and can solve problems such as the variation of the YMDD functional region of the virus polymerase P protein, virus drug resistance, and inability to cure hepatitis B
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Screening of inhibitory peptides for the YMDD functional region of hepatitis B virus polymerase protein
[0023] (1) Synthesis of short peptides in the YMDD functional region of hepatitis B virus polymerase protein: Designed according to the HBV ayw genotype sequence (genbank access no: U95551), synthesized short peptides in Hangzhou Zhongpi Biochemical Co., Ltd. The sequence is: Arg Arg Ala PhePro His Cys Leu Ala Phe Ser Try Met Asp Asp Val Val Leu Gly Ala. The purity of the short peptide is> 85%, and the solubility is> 0.5 mg / ml.
[0024] (2) Screening of phage peptide library
[0025] 1. The first day:
[0026] 1.1. With 0.1M NaHCO 3 (PH8.6) Prepared target molecule solution: prepared by short peptide of YMDD functional region of hepatitis B virus polymerase protein DMSO solution first, then add 0.1M NaHCO 3 (PH8.6) dissolved.
[0027] 1.2. Coating: add in each hole The target molecule solution, vortex repeatedly until the surface is completely wetted. In a ...
Embodiment 2
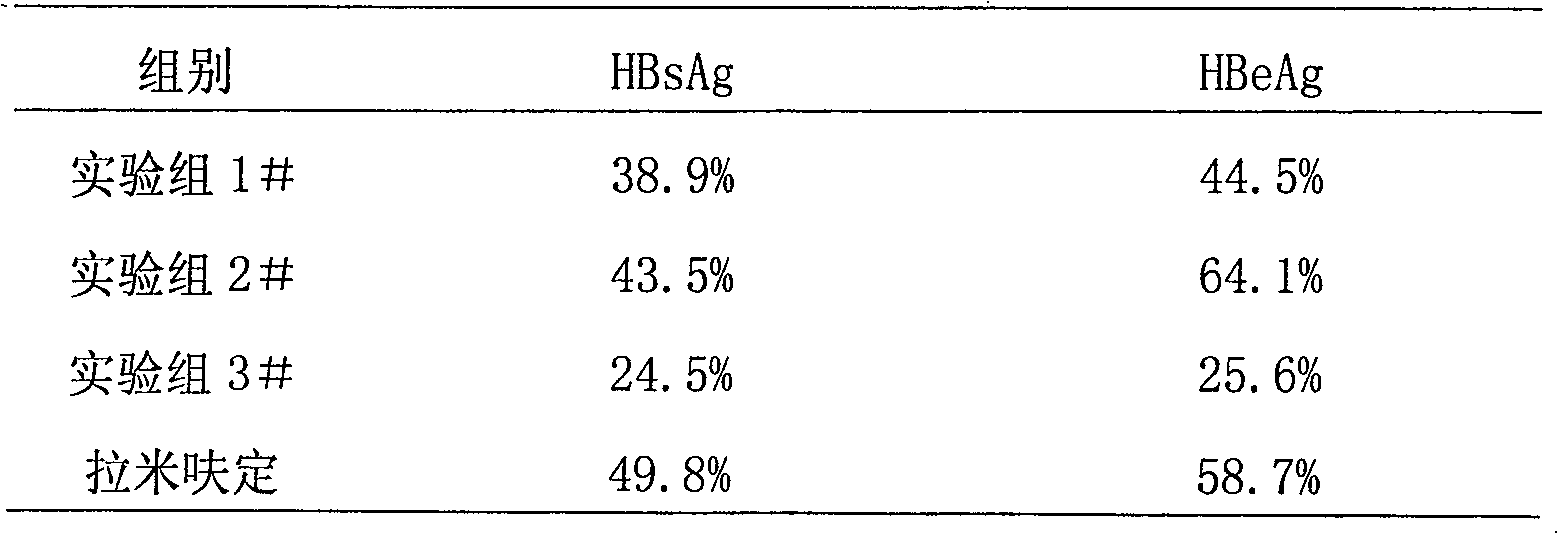
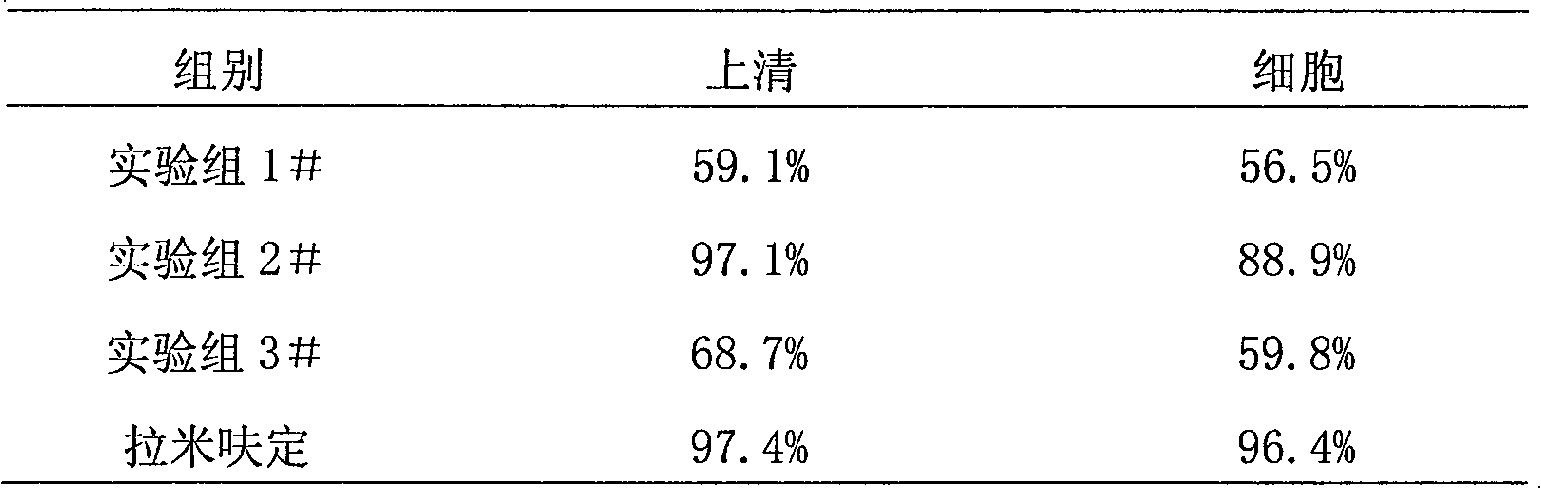
[0085] Example 2 Experiment of inhibitory peptide on the inhibitory effect of hepatitis B virus
[0086] (1) Cell culture
[0087] 1. Cultivation of 2.2.15 cells 2.2.15 cells are hepatocellular carcinoma cell lines transfected with full HBV genes, containing polyploidy of complete HBV genes, capable of transcribing and translating HBV genes and producing and secreting HbsAg, HbeAg, HBV DNA and HBV Particles. It was cultured under pressure with RPMI1640 medium, 10% newborn calf serum, 400mg / L G418. 2.2.15 cells press 2×10 6 / Plate inoculated on a 60mm cell culture plate. The experimental group consists of synthetic peptides (Trp Try Thr Asn Asn Ser Thr (HBV YMDD functional area inhibitory peptide 1#), Gly Pro Phe Asn Asn Pro Pro (HBV YMDD functional area inhibitory peptide 2#), Gly Trp Leu Pro ProPro Asp( HBV YMDD functional domain inhibitory peptide 3#), synthesized by Hangzhou Zhongpi Biochemical Co., Ltd.), added per plate Inhibitory peptide solution. Lamivudine control group p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com