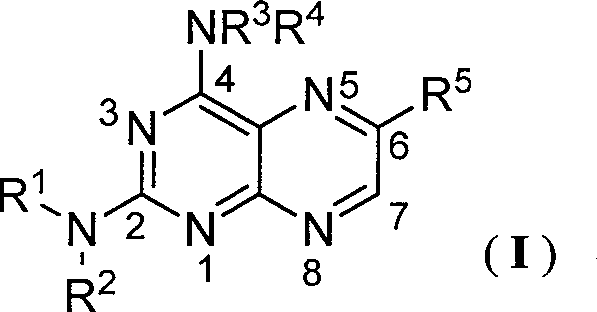
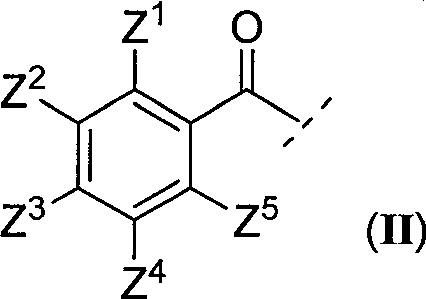
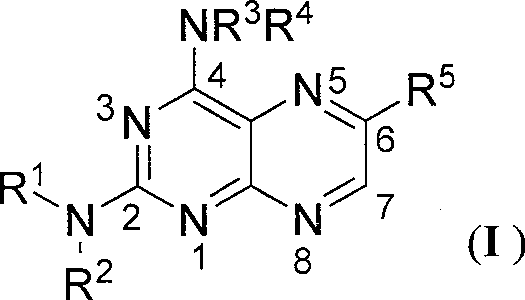
N2-arylformyl-4-ammonia/amidopteridine compound capable of regulating nitrous oxide synthase acitivity
A technology of aroyl and compound, applied in the field of N2-aroyl-4-amine/amino-pteridine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] 1 Compound preparation
[0043] 1.1 Intermediate 2,6-diamino-4-diethylaminopyrimidine (III-1)
[0044] Add 2,6-diamino-4-chloro-5-p-chlorobenzopyrimidine (5.00g, 17.7mmol) into 20ml of DMF, then add diethylamine (10g, 0.137mol), heat to 70°C and stir the reaction 5h, then poured into ice water, a yellow solid precipitated, filtered, washed with water, and dried to obtain 4.01g of yellow powder 2,6-diamino-4-diethylamino-5-p-chlorobenzopyrimidine, yield: 71%, m.p: 148~150℃, compared with literature (Frohlich LG, Kotsonis P, Traub H, et al. Inhibition of neuronal nitricoxide synthase by 4-amino pteridine derivatives: Structure-activity relationship of antagonists of (6R)-5, 6 , 7, 8-tetrahydrobiopterin cofactor. J Med Chem, 1999, 42: 4108-4121) the results were consistent.
[0045] 2,6-diamino-4-diethylamino-5-p-chlorophenylazopyrimidine (1.28g, 4mmol) was added to 20ml of ethanol, 15ml of 5% HCl solution and the mixed solution, and the temperature was raised to 70°C ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com