2, 4-diamido-6-pteridine methyl methylamino aminobenzene benzoyl-Glu-peptide, and compound, activity and application thereof
A technology of pteridylmethyl and methylamino, which is applied in the field of biomedicine and can solve problems such as unsatisfactory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
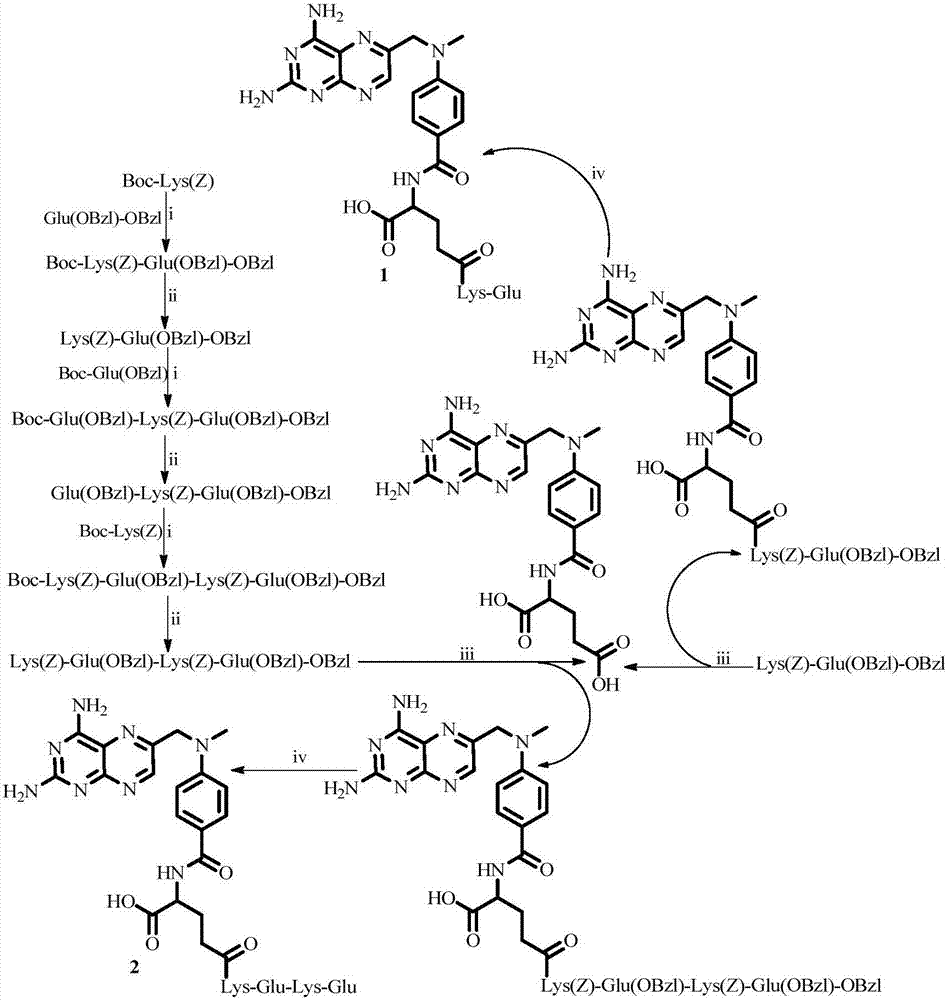
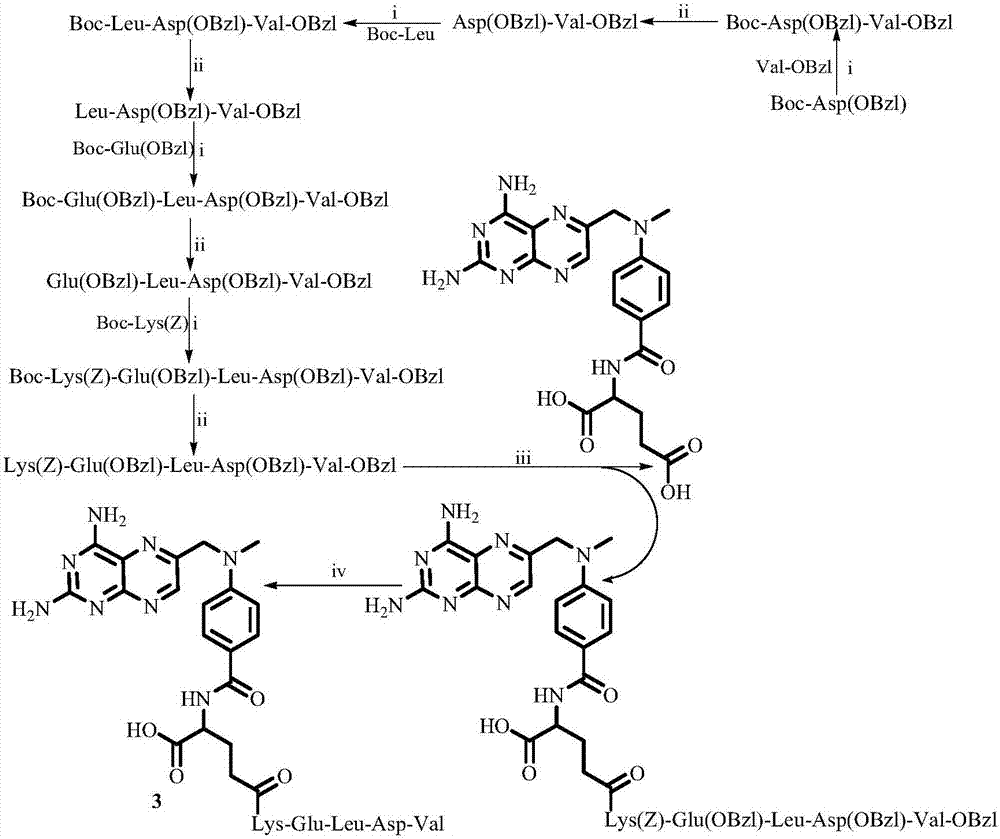
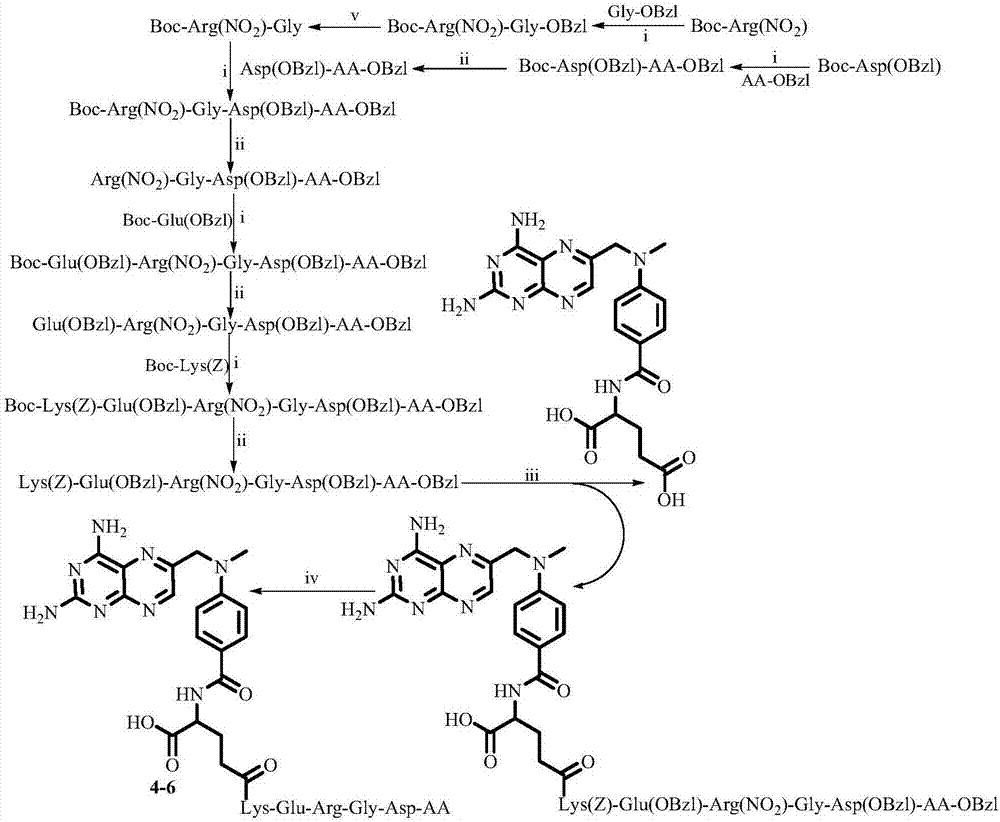
[0094] Example 1 Preparation of Boc-Lys(Z)-Glu(OBzl)-OBzl
[0095] 3.80g (10mmol) Boc-Lys (Z) was dissolved in 20mL of anhydrous tetrahydrofuran (dry THF) in a 250mL reaction flask, and 2.47g (12mmol) of N,N-dicyclohexylcarbodiimide (DCC) was added under ice cooling and 0.67g (5mmol) N-hydroxybenzotriazole (HOBt), stirred and activated for 30min to obtain reaction solution A; in addition, 3.27g HCl Glu(OBzl)-OBzl was dissolved in 50mL reaction flask with 20mL dryTHF, and ice bath Slowly add N-methylmorpholine (NMM) dropwise to adjust the pH value to 8 to obtain a reaction solution B. Under an ice bath, add the reaction solution B to the reaction solution A, slowly add NMM dropwise to adjust the pH value to 9, at room temperature Under stirring reaction 12h, thin layer chromatography TLC (dichloromethane:methanol=15:1) shows that reaction is finished, and reaction solution is filtered, and desolvent is removed by concentration under reduced pressure, and residue is separated by...
Embodiment 2
[0096] Example 2 Preparation of HCl Lys(Z)-Glu(OBzl)-OBzl
[0097] 3.72g (5.40mmol) Boc-Lys(Z)-Glu(OBzl)-OBzl was added to a 250mL reaction flask, dissolved with 25mL of anhydrous ethyl acetate, and 54mL of 4N HCl / EA solution was added under ice cooling, and the reaction was stirred for 1.5 h, thin-layer chromatography TLC (dichloromethane: methanol = 15:1) showed that the reaction was complete, the reaction solution was decompressed to remove the solvent, and anhydrous ether was added to remove the solvent and HCl gas under reduced pressure again to obtain 2.86g (90.0%) The title compound as a white solid. ESI-MS(m / e):590[M+H] + .
Embodiment 3
[0098] Example 3 Preparation of Boc-Glu(OBzl)-Lys(Z)-Glu(OBzl)-OBzl
[0099] According to the method of Example 1, by 1.63g (4.86mmol) Boc-Glu (OBzl), 1.20g (5.83mmol) DCC, 0.67g (2.43mmol) HOBt, and 2.86g (4.86mmol) HCl Lys (Z) -Glu(OBzl)-OBzl afforded 2.30 g (52.3%) of the title compound as a white solid. ESI-MS(m / z):909[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com