Powder injection contg high content tanshin polyphenolic acid salts, and its preparation method
A technology of salvia polyphenolate and polyphenolate, which is applied in the directions of medical preparations containing active ingredients, powder delivery, pharmaceutical formulations, etc., can solve problems such as adverse reactions, occurrences, and incomplete elucidation of the active ingredients of salvia miltiorrhiza
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
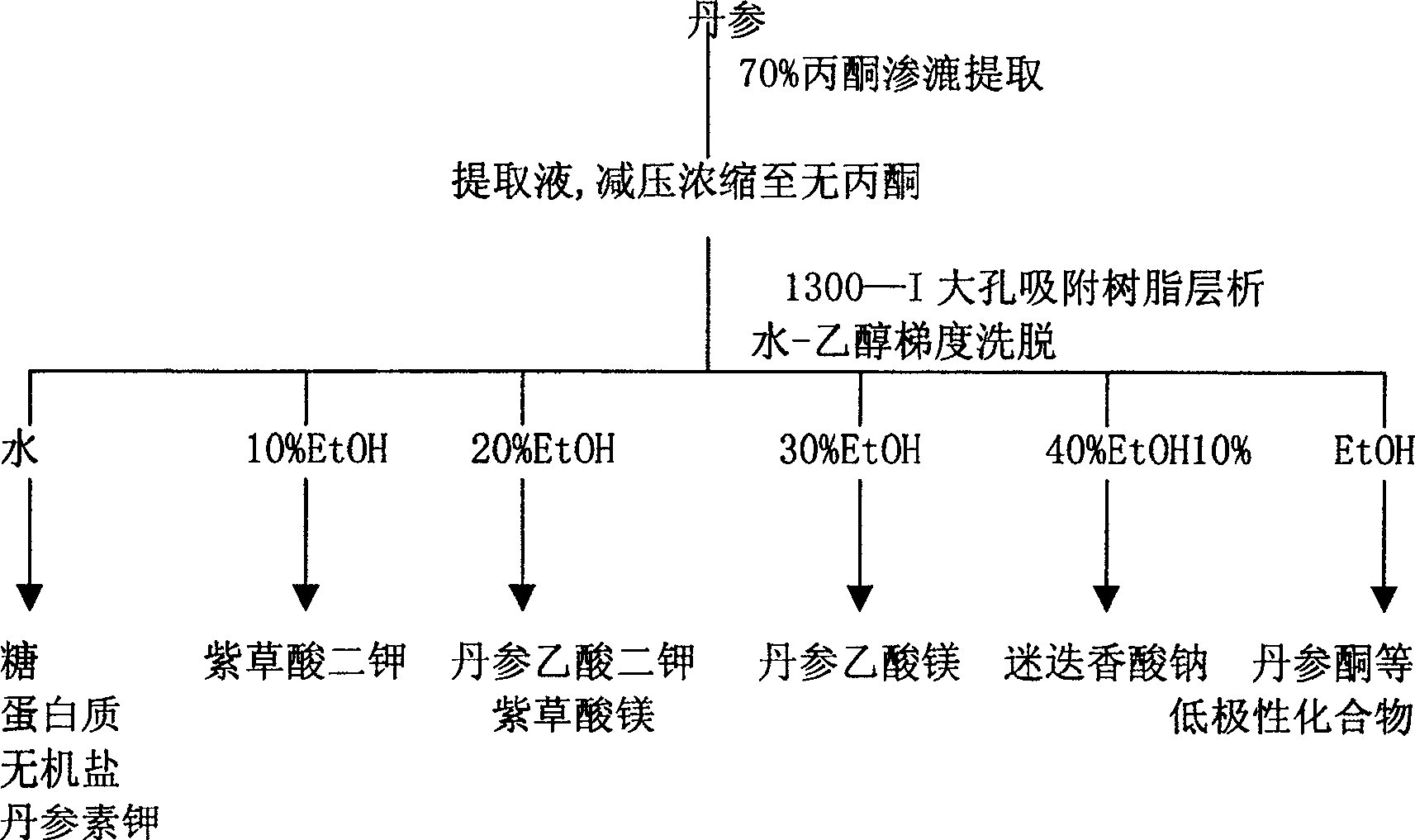
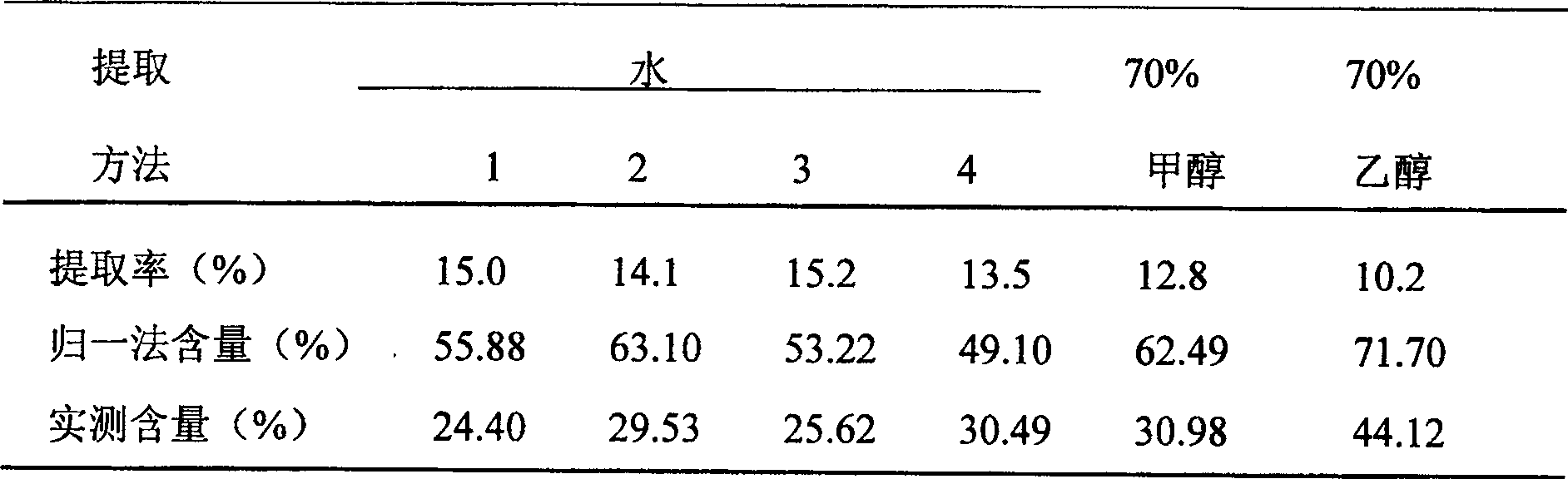
[0067] After 1 portion of Salvia miltiorrhiza is crushed, it is decocted and extracted three times with 4 times the amount of water, 2 times the amount of water, and 2 times the amount of water, and the extraction time is 2 hours each time. The three extracts were combined, cooled to room temperature, centrifugally filtered, and the filtrate slowly flowed through an adsorption column equipped with 3 times the amount of 1300-I macroporous resin. After the adsorption is complete, wash away sugar, protein and inorganic salts with 6 times the amount of water, then use 6 times the amount of 20% ethanol to remove salvianolate compounds other than Salvia acetic acid salt, and finally wash with 3 times the amount of 50% ethanol Desalvianolate. According to TLC detection, collect 50% ethanol eluate rich in salvia miltiorrhiza, concentrate under reduced pressure to 1 / 10 of the crude drug amount, slowly add 9 / 10 of the crude drug amount of absolute ethanol under stirring, and let it stan...
example 2
[0069] After 1 portion of Salvia miltiorrhiza is crushed, it is decocted and extracted three times with 4 times the amount of water, 2 times the amount of water, and 2 times the amount of water, and the extraction time is 2 hours each time. The three extracts were combined, cooled to room temperature, centrifugally filtered, and the filtrate slowly flowed through an adsorption column equipped with 3 times the amount of D101 macroporous resin. After the adsorption is complete, wash away sugar, protein and inorganic salts with 6 times the amount of water, then use 6 times the amount of 20% ethanol to remove salvianolate compounds other than Salvia acetic acid salt, and finally wash with 3 times the amount of 50% ethanol Desalvianolate. According to TLC detection, collect 50% ethanol eluate rich in salvia miltiorrhiza, concentrate under reduced pressure to 1 / 10 of the crude drug amount, slowly add 9 / 10 of the crude drug amount of absolute ethanol under stirring, and let it stand ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com