Chitin sulfanilamide derivative and its production
A technology of chitosan and derivatives, applied in the field of chitosan and its C-6 sulfuric acid ester sulfonamide derivatives and its preparation, can solve the problems of poor effect, achieve good water solubility and enhance antibacterial Active, wide-ranging effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take 2g with HAc-H 2 o 2 The chitosan with a degraded molecular weight of 4,000 has a deacetylation degree of 80%. Dissolve in 100 mL of dimethyl sulfoxide, add 5.78 g of p-acetamidobenzenesulfonyl chloride dissolved in 20 mL of dimethyl sulfoxide under stirring, and react at 65°C for 3 hours. After cooling, pour the reaction mixture into 300mL acetone to obtain a precipitate, filter and wash the precipitate with acetone, add 100mL distilled water to dissolve, put it into a dialysis bag with a molecular weight of 3600, dialyze with distilled water for 2-3 days, and concentrate to about 30mL by rotary evaporation. Chitosan sulfonamide derivatives (as shown in Formula 1, wherein n=25) were obtained after freeze-drying, wherein the degree of substitution of sulfonamide groups was 40.2%.
[0029]
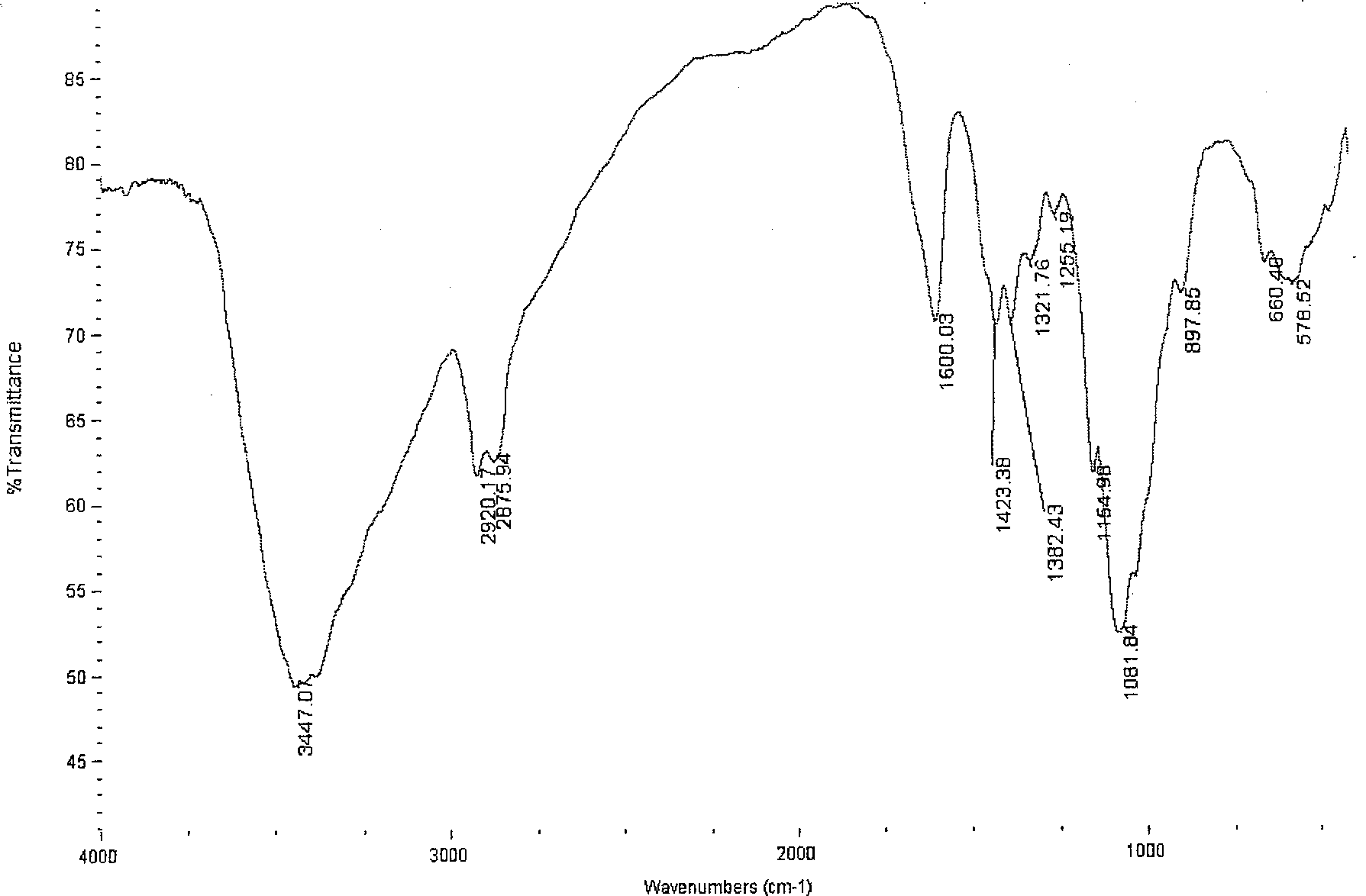
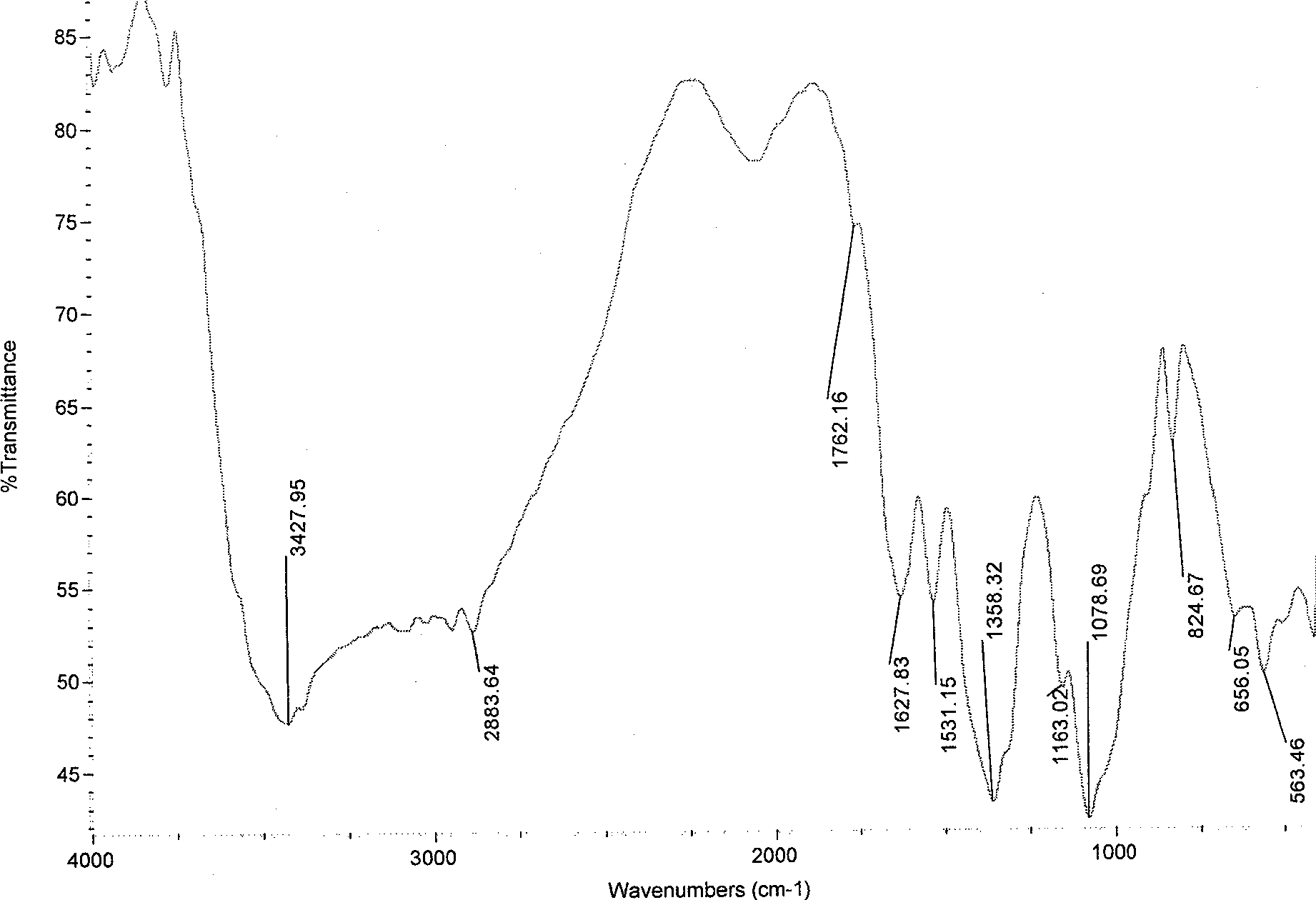
[0030] Infrared spectroscopy analysis showed that (see image 3 ), chitosan sulfonamide derivatives and chitosan (see figure 1 ) compared with 1358.32, 1163.02 and 824.67 ...
Embodiment 2
[0032]2g chitosan, its molecular weight is about 200,000, the degree of deacetylation is 96%, dissolve in 80mL formamide, add 2.89g p-acetamidobenzenesulfonyl chloride dissolved in 20mL formamide under stirring, and react at 75°C for 3 hours . After cooling, pour the reaction mixture into 200 mL of absolute ethanol to obtain a precipitate, filter and wash the precipitate with acetone, add 100 mL of distilled water to dissolve, put it into a dialysis bag with a molecular weight of 3600, dialyze with distilled water for 2-3 days, and concentrate to 30 mL by rotary evaporation Left and right, chitosan sulfonamide derivatives are obtained after freeze-drying, the structure is shown in formula 1, wherein n=1242.
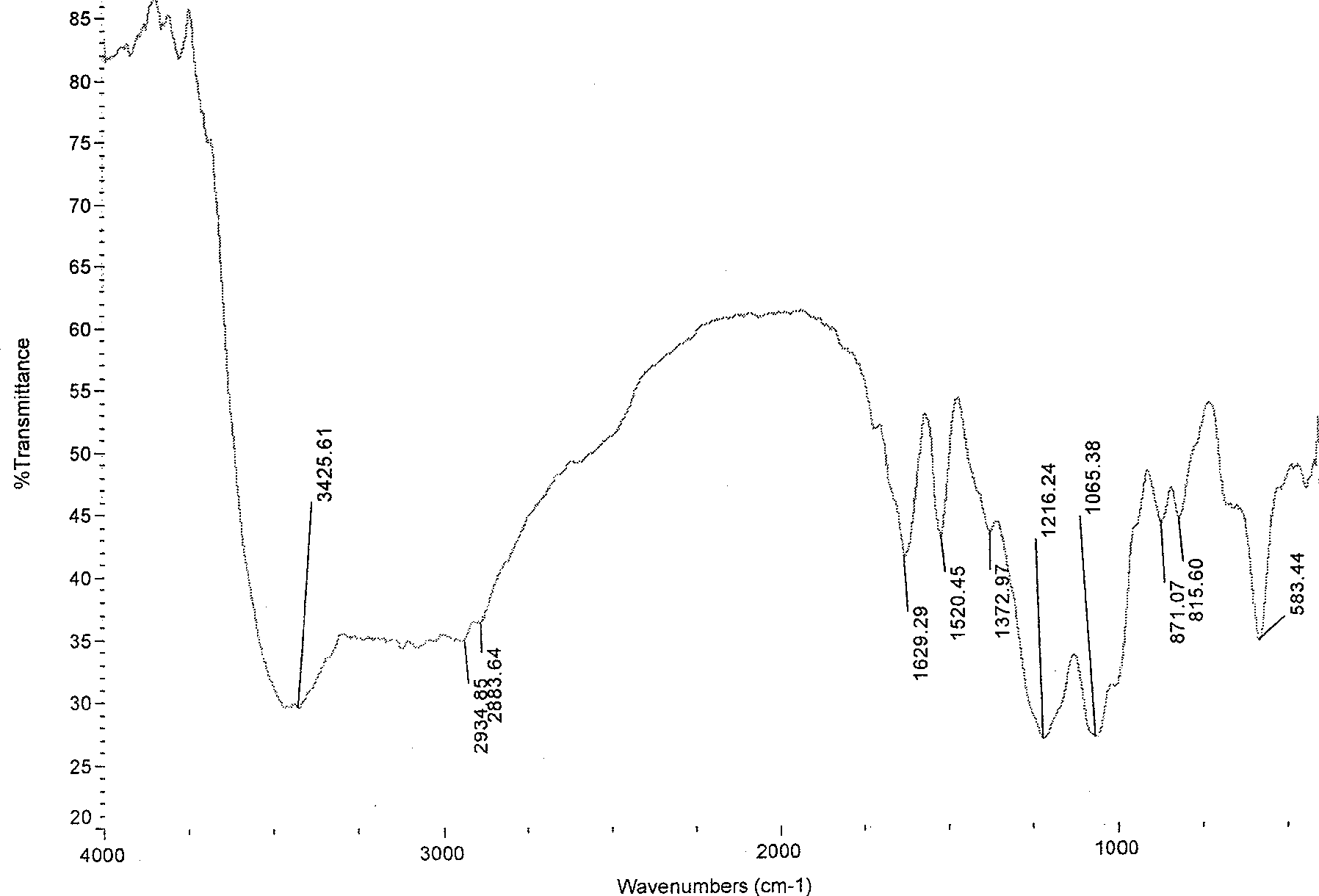
[0033] Infrared spectroscopy analysis showed that (see Figure 4 ), chitosan sulfonamide derivatives and chitosan (see figure 1 ) compared with 1376.69, 1166.54 and 836.15 three new absorption peaks, which are the characteristic absorption peaks of the sulfonamide group...
Embodiment 3
[0035] Chitosan sulfate used as raw material is prepared from chitosan by known methods, such as using chitosan and sulfonating reagent DMF-SO 3 The reaction gives chitosan sulfate. (Preparation of high-molecular weight and high-sulfate content chitosans and their potential antioxidant activity in vitro, Ronge Xing; Song Liu; HuahuaYu; Zhanyong Guo; Zhien Li; Pengcheng Li, Carbohydrate Polymers, 2005, 61(2), 148-154. ) Get 2g of chitosan sulfate, its molecular weight is 09,000, and the S content is 10.55%. Dissolve in 50 mL of dimethyl sulfoxide, add 2.91 g of p-acetamidobenzenesulfonyl chloride dissolved in 20 mL of dimethyl sulfoxide under stirring, and react at 65°C for 4 hours. After cooling, pour the reaction mixture into 300mL acetone to obtain a precipitate, wash the precipitate with acetone after filtration, add 50mL distilled water to dissolve, put it into a dialysis bag with a molecular weight of 3600, dialyze with distilled water for 2-3 days, and concentrate to ab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com