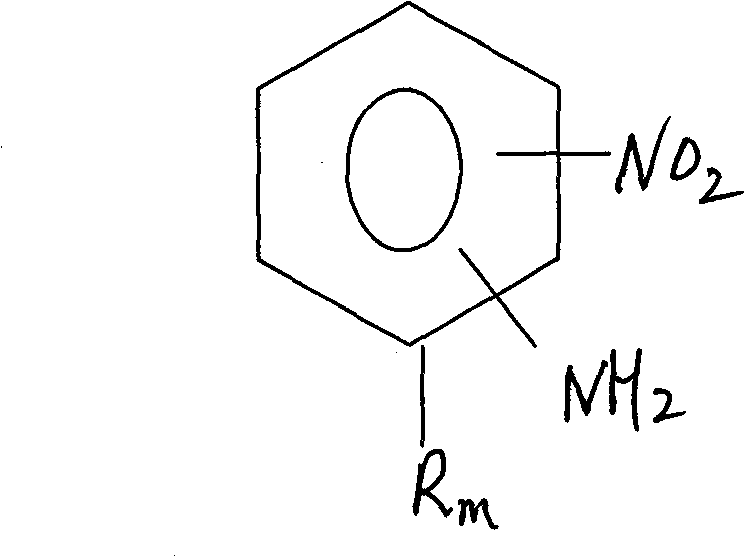
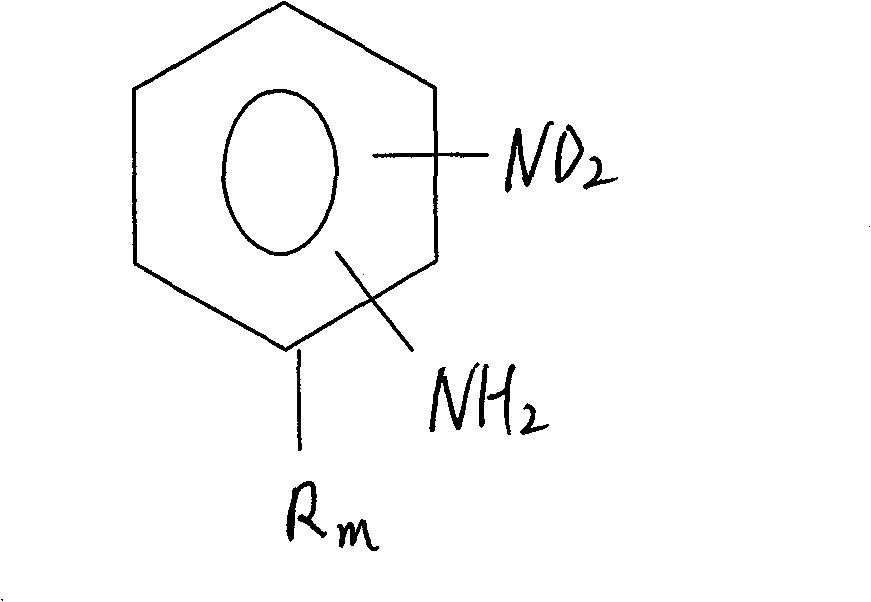
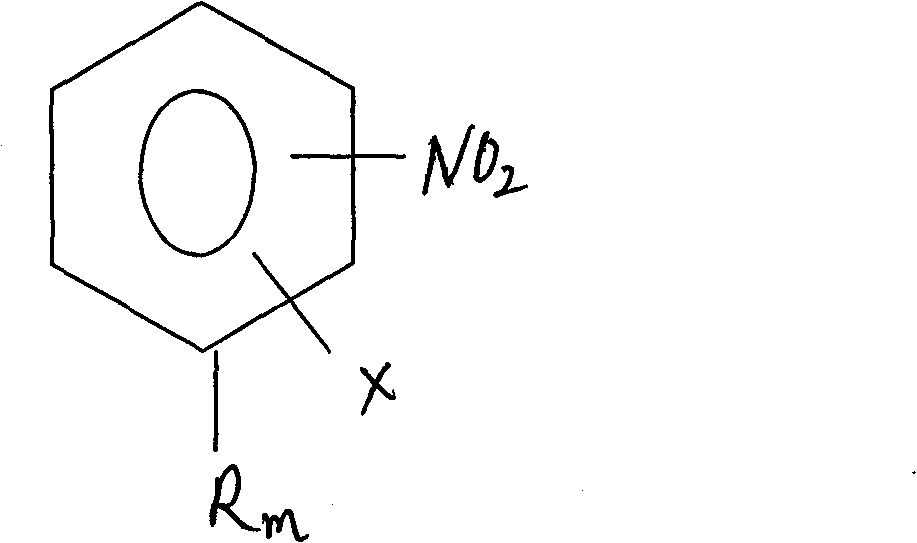Process for preparing relevent aniline kind compound of ammonolysis of partial nitro benzene halide kind compound
A technology of aniline compounds and nitrohalobenzene, which is applied in the field of preparation of organic compounds, can solve problems such as poor safety, low product purity, and excessive sulfur-containing waste water in waste gas, and achieve good economic and social benefits, process simplification, The effect of less investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 34.2g of 4-chloro-2-nitrotoluene and 100g of water into a 1L stainless steel autoclave, add 100g of liquid ammonia under airtight conditions; then raise the temperature and pressurize to 200-240°C, 5-6MPa, and react for 14 hours . According to HPLC analysis, the raw material peak disappeared, cooled to 30°C, filtered, washed with water, purified, and dried to obtain 23.6g of 4-amino-2-nitrotoluene with a purity of 99.0% and a yield of 83.6%. Ammonia water is recovered for reuse.
Embodiment 2
[0022] Add 34.2g of 6-chloro-2-nitrotoluene, 150g of 18% ammonia water recovered in Example 1, and 1g of CuCl into a 1L stainless steel autoclave, add 34.5g of liquid ammonia under airtight conditions, heat up and pressurize to 200- 240°C, 5-6MPa, react for 10 hours. According to HPLC analysis, the raw material peak disappeared, cooled to 30°C, filtered, washed with water, and dried to obtain 25.7g of 6-amino-2-nitrotoluene with a purity of 99.2% and a yield of 91.1%.
Embodiment 3
[0024] Add 34.2g of 6-chloro-2-nitrotoluene, 100g of water, 1g of CuCl and 1g of tetrabutylammonium bromide into a 1L stainless steel autoclave, add 100g of liquid ammonia under airtight conditions, raise the temperature and pressurize to 200-240 ℃, 5~6MPa, react for 7 hours. According to HCLP analysis, the raw material peak disappeared, cooled to 30°C, filtered, washed with water, and dried to obtain 26.1g of 6-amino-2-nitrotoluene with a purity of 99.3% and a yield of 92.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com