Triphenylamine derivative double-photon optical storage material and preparation method thereof
A technology of triphenylamine and derivatives, which is applied in the field of triphenylamine derivative two-photon optical storage materials and its preparation, can solve the problems that have not been implemented, the molecular absorption cross section of two-photon initiators is small, and it is difficult to meet the requirements of practical applications. Achieve the effects of small synthesis and separation workload, increased two-photon absorption cross section, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
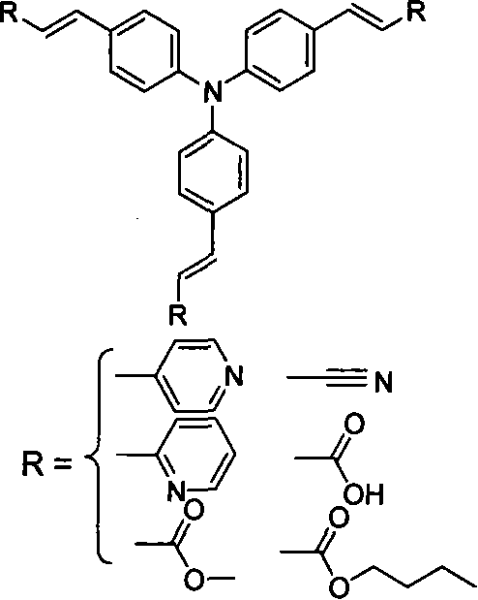
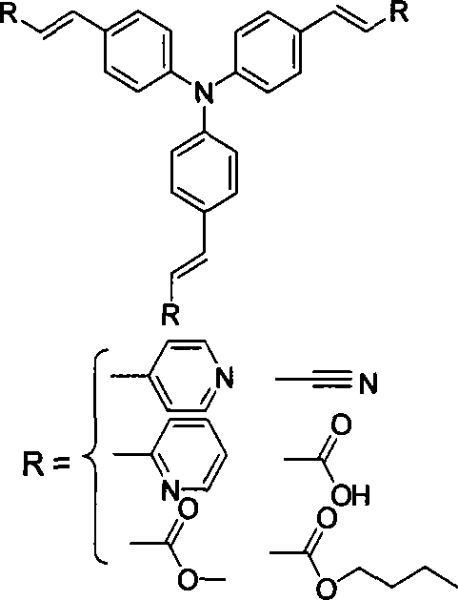
[0020] Take R as an example for the synthesis of 4-vinylpyridine to synthesize three-(4-pyridylvinylphenyl)amine, and the non-limiting examples are described as follows:
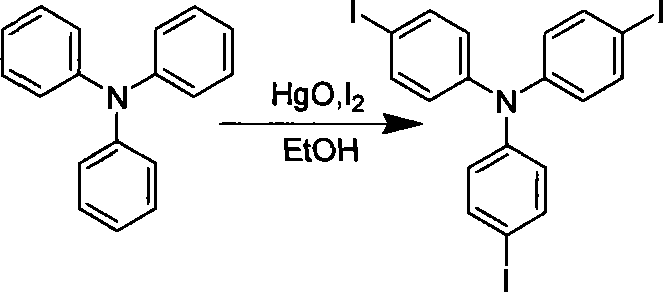
[0021] 1. Preparation of intermediates
[0022]
[0023] Add 1.0g (4mmol) triphenylamine to 50mL of absolute ethanol, after stirring and dissolving at room temperature, mix 4.06g (18.6mmol) HgO and 5.08g (20mmol) I 2 Add in small amounts sequentially, stir vigorously for about 20 hours, evaporate ethanol to dryness, add 30mL of toluene to reflux, and pass the solution directly through a short Al 2 o 3 The sand core funnel, repeated four times. Most of the toluene was distilled off, and a large amount of red solid was precipitated after cooling slightly. After washing with hot methanol under reflux for three times, 2.3 g of white needle-like crystals were obtained, with a yield of 92%.
[0024] 2. Synthesis of target compounds
[0025]
[0026] At room temperature, 2.07g (15mmol) of potassium carbon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com