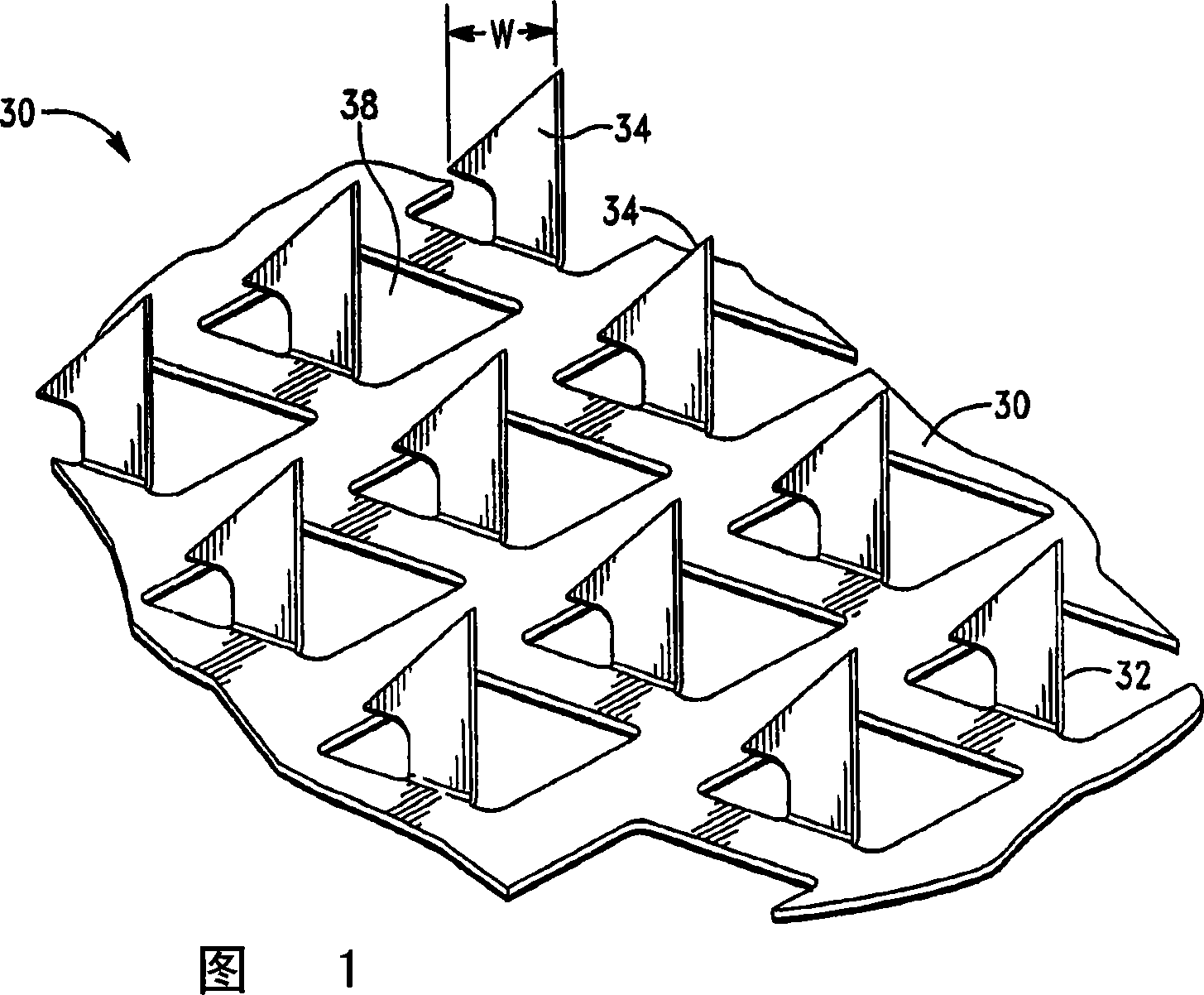
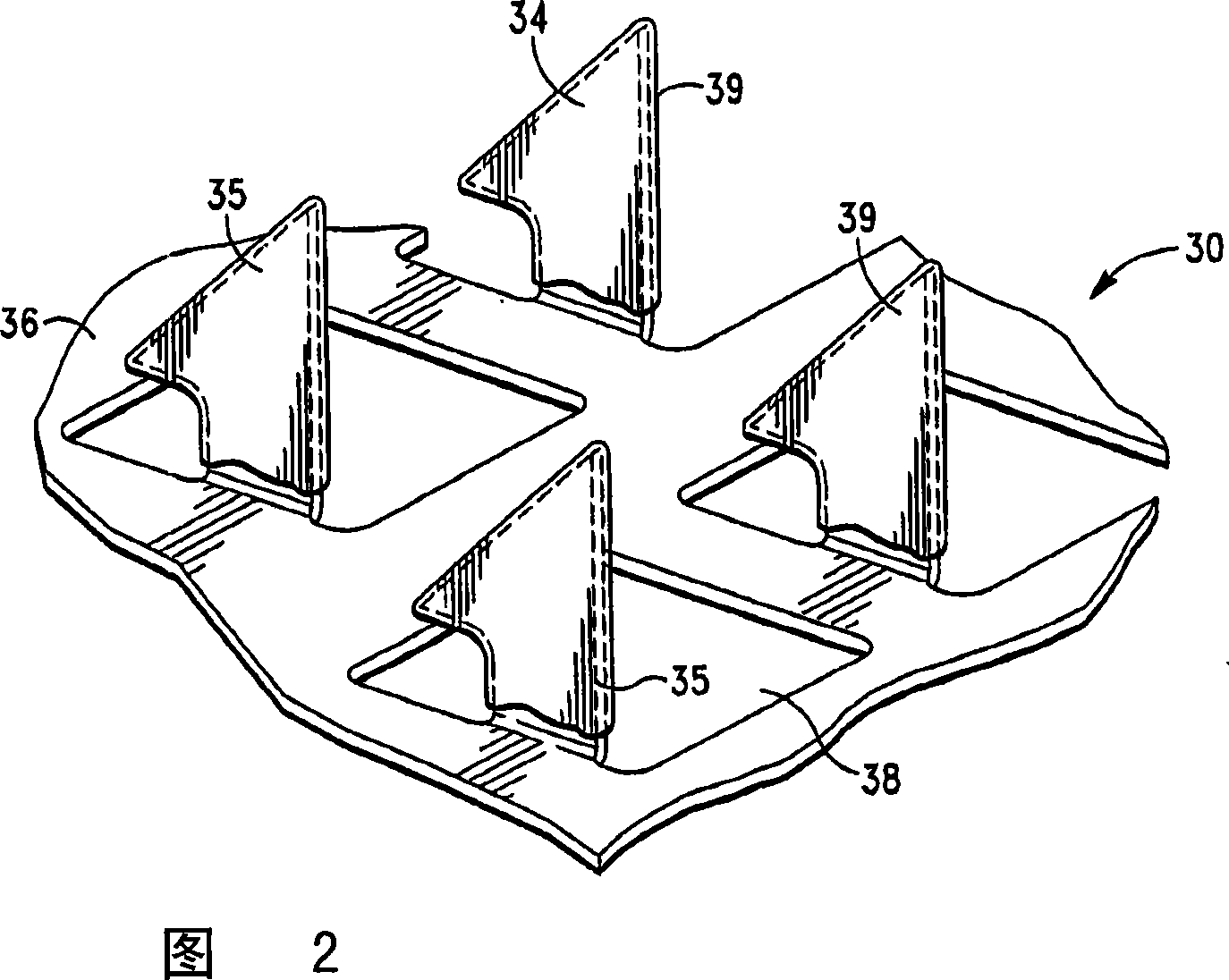
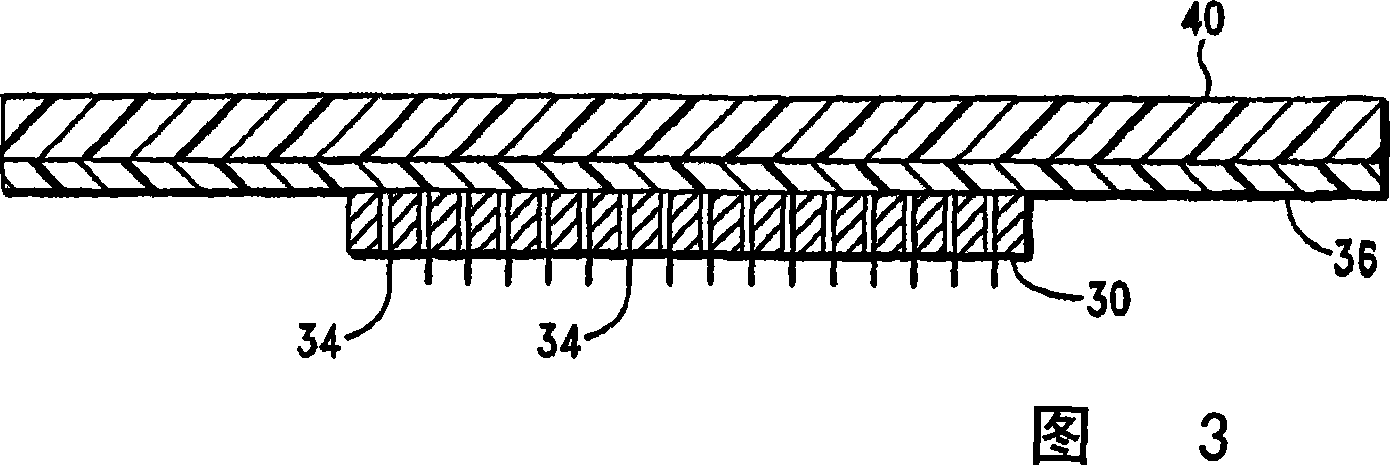
Microprojection array patch for transdermal delivery of vascular endothelial growth factors
A technology of micro-protrusions and protruding elements, which is applied in the directions of drug delivery, sheet-like delivery, and devices introduced into the body, etc., can solve problems such as limited efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0265] The following examples are given to enable those skilled in the art to more clearly understand and apply the present invention. They are not to be considered as limitations on the scope of the invention, but merely to represent typical embodiments thereof.
VEGF preparations
[0266] The following studies were performed to develop suitable assays and demonstrate the suitability of VEGF-based reagent formulations for coating transdermal delivery microprojection arrays. VEGF was obtained from Scios in a volume of 110 mL (Scios lot #8331:058 rework #1). 100 mL of this solution (@1.82 mg VEGF / mL) was prepared in a 3:1 weight ratio of sucrose and VEGF, and packed into four 50 mL centrifuge tubes, each containing 25 mL of liquid formulation. After lyophilization, each powder was reconstituted with 2 mL of WFI, mixed and loaded into a 10000 MWCO dialysis card. The concentrate was exchanged twice in 1 L against 2 mM citrate buffer (pH 4.6) at 2-8° C., at least 12 h per exch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com