Use of IL-18 inhibitors
1. The technology of IL-18 and inhibitors, applied in the fields of treatment and/or prevention of arthritis, treatment and/or prevention of inflammatory bowel disease, treatment and/or prevention of liver diseases, can solve problems such as effect doubts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Example 1: Production of IL-18BP-His tag
[0169] Purified recombinant human IL-18BP containing a histidine tail (r-hIL-18BP-His tag) was produced in CHO cells. Those skilled in the art know that recombinant proteins can be produced in eukaryotic cells. Well-known methods can be used to construct appropriate vectors carrying DNA encoding IL-18BP, which are suitable for transfection of eukaryotic cells to produce recombinant IL-18BP. For cellular expression, the DNA encoding IL-18BP (see Novick et al., 1999) was excised and inserted into an expression vector suitable for transfected cells. Alternatively, this DNA can be prepared by PCR with appropriate sense and antisense primers. The resulting cDNA construct is then inserted into an appropriately constructed eukaryotic expression vector using techniques well known in the art (Manaitis, 1982). The recombinant protein is purified to a purity of more than 95%, and it is found that it has biological activity in vivo and ...
Embodiment 2
[0170] Example 2: Protective effect of IL-18BP on endotoxin-induced death mouse model
[0171] A mouse model was used to examine whether IL-18BP (IL-18 inhibitor) could protect mice against high doses of lipopolysaccharide (LPS). Mice died rapidly after LPS-induced acute liver injury.
[0172] C57BL / 6 mice were injected intraperitoneally (ip) with his-tailed recombinant human IL-18BP (rhIL18BPhis, obtained from recombinant production of the protein) at 4 mg / kg, 1 hour later, with 60 mg / kg of LPS (lethal dose) , Survival rate of mice was compared with the group of animals receiving LPS only (no IL-18BP).
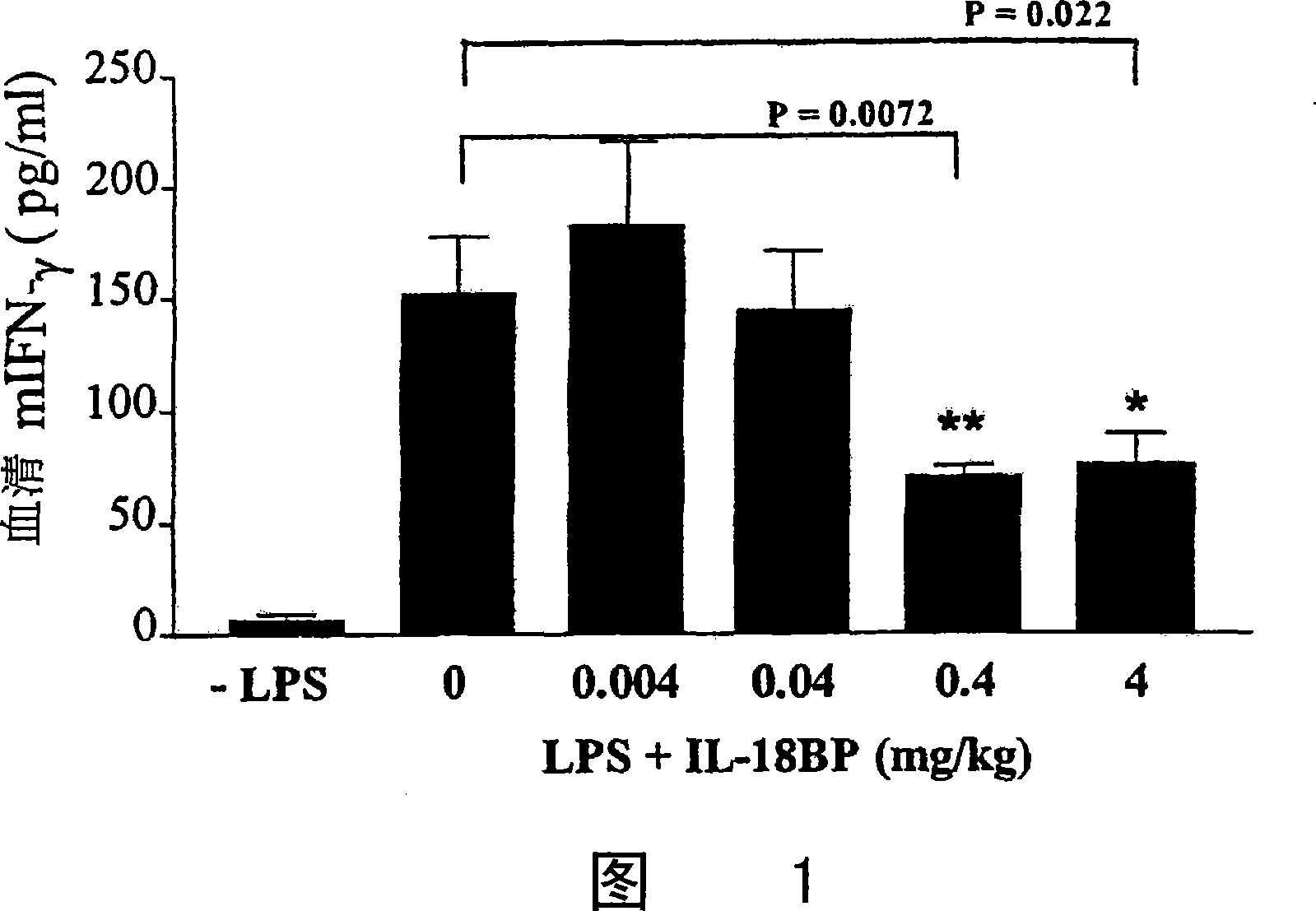
[0173] Five out of seven mice injected with rhIL-18BP-his survived LPS injection compared with control mice, while all mice in the control group died within three days.
[0174] In the absence or presence of increasing doses of rhIL-18BP-his, blood samples were collected 5 hours after LPS injection for ELISA analysis of circulating IFN-γ (Fig. 1). 0.4 and 4 mg / kg rhIL-18BP...
Embodiment 3
[0175] Example 3: IL-18BP has a protective effect against liver injury in a mouse pathological model
[0176] A mouse model of fulminant hepatitis was used to test the effect of IL-18BP. When sequentially administered Proplioni-bacterium acnes (P.acnas) and lipopolysaccharide (LPS), mice developed liver injury.
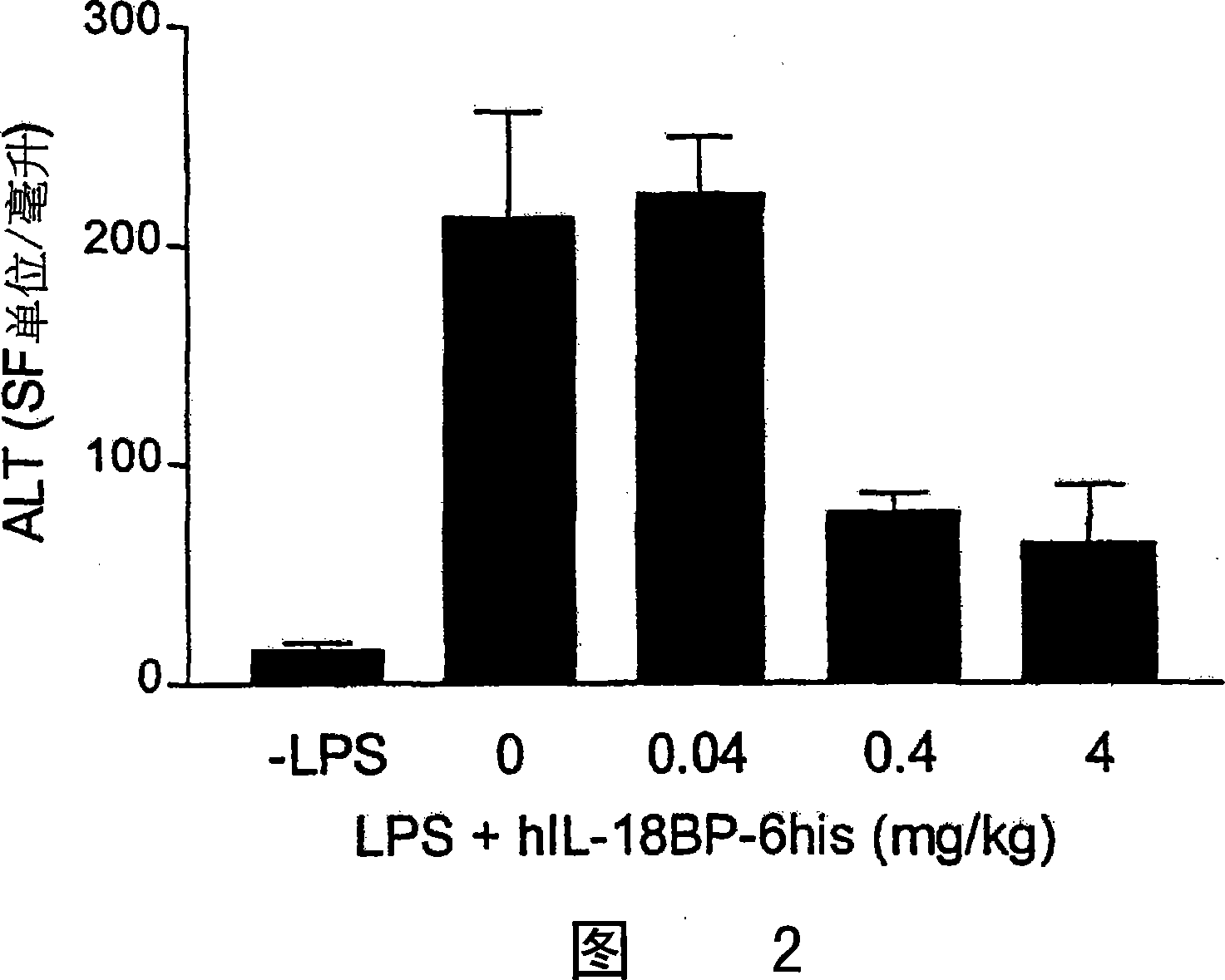
[0177] P.acnas-sensitized C57BL / 6 mice were injected with increasing doses of rhIL-18BP-his (4, 0.4, 0.04, 0 mg / kg) at different times (1 hour, 20 minutes, simultaneously) before LPS injection. No mice survived and circulating IFN-γ and TNF-α levels were unaffected when rhIL-18BP-his was administered ip concurrently with LPS. Surprisingly, rhIL-18BP (4 and 0.4 mg / kg) resulted in a 70% reduction in circulating alanine aminotransferase, a marker of liver damage, as shown in FIG. 2 .
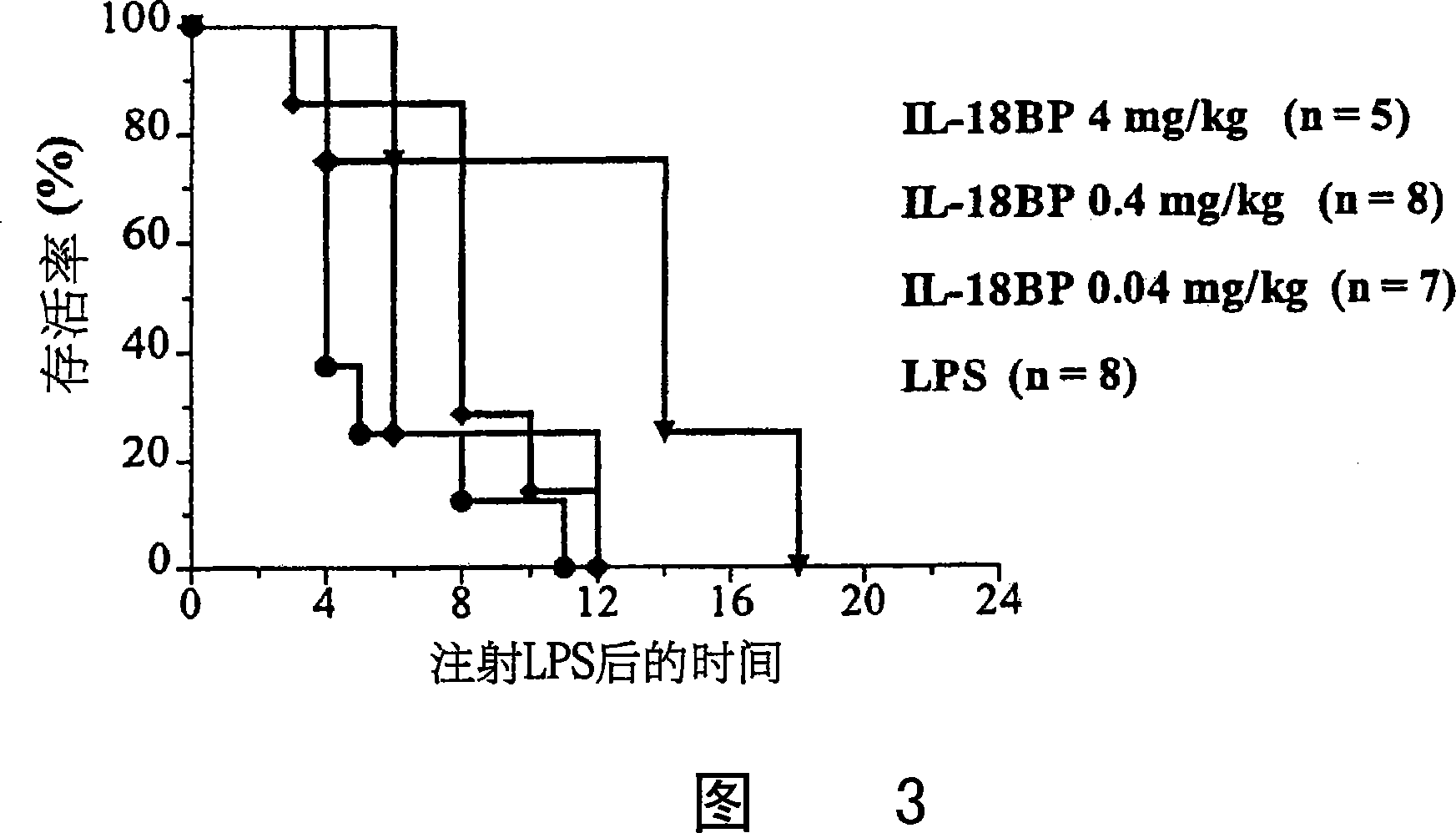
[0178] In addition, mouse survival was monitored (Fig. 3), when rhIL-18BP was administered ip 20 minutes before LPS, compared with control mice (receiving NaCl instead of IL-18BP), the t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com