Imine compound activated iron polyolefin catalyzer
A technology of polyolefin catalysts and compounds, applied in the field of iron-based polyolefin catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
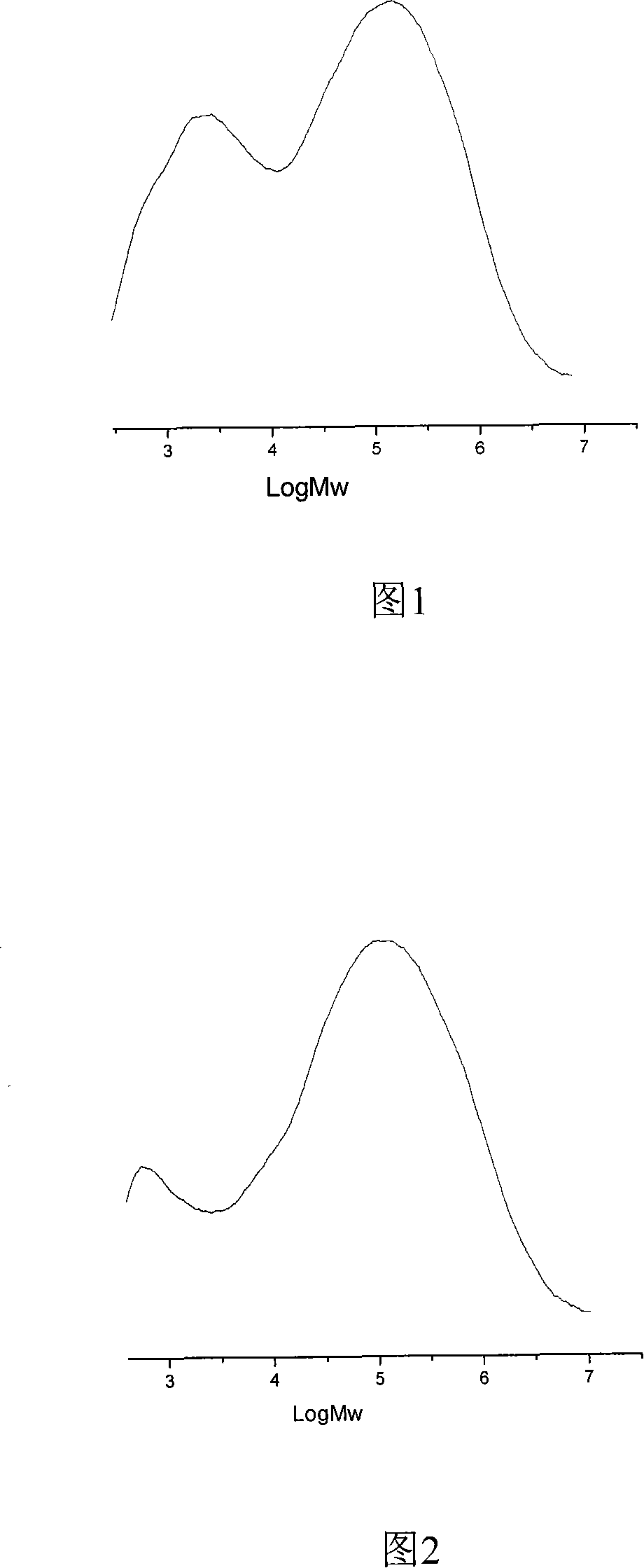
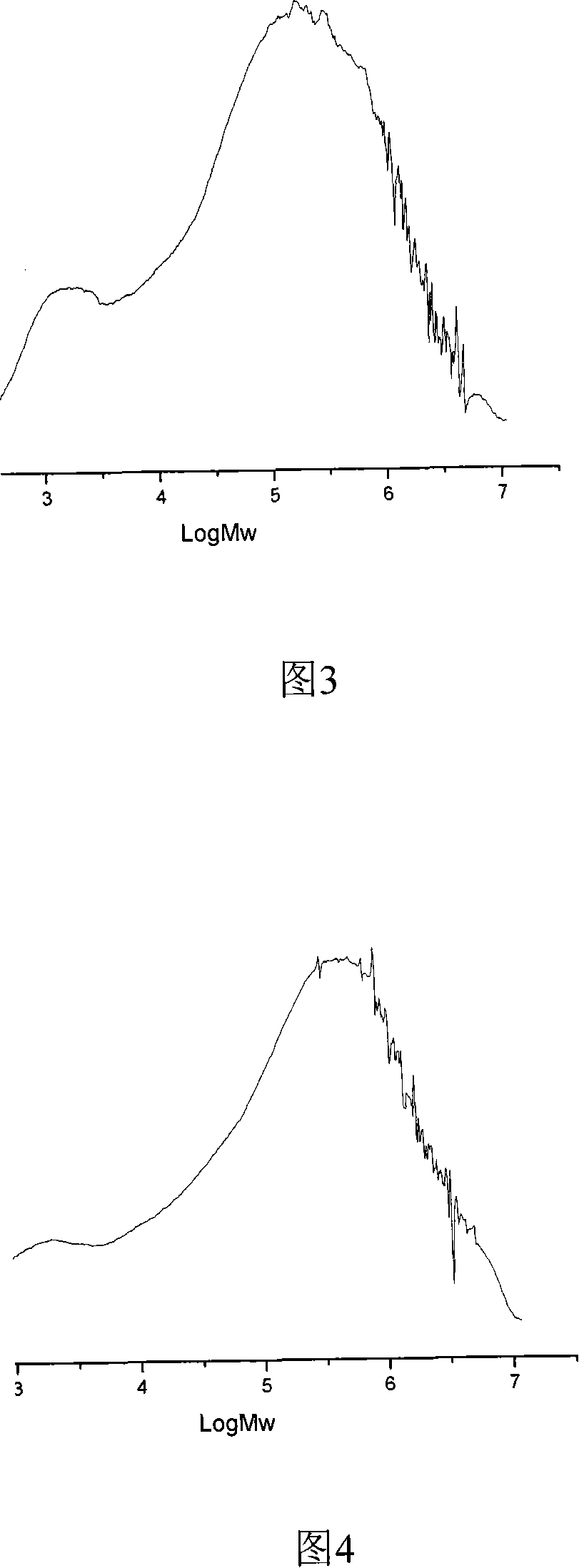
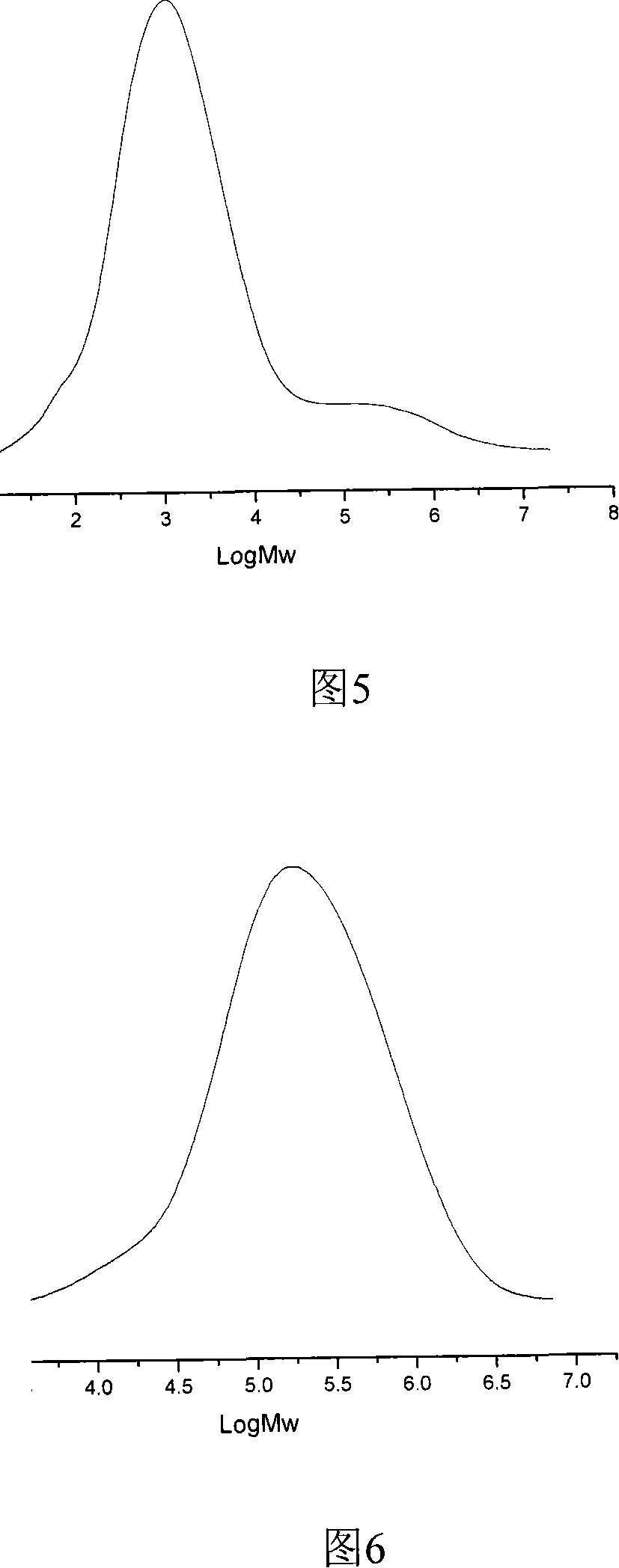
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 Schiff base
[0037]
[0038] With 30mL absolute ethanol as solvent, add 2,6-dimethylaniline (1.94g, 16.0mmol) and 2,6-diacetylpyridine (2.61g, 16.0mmol) successively, add dropwise 1mL formic acid as catalyst, room temperature Under stirring for 48h. A yellow precipitate appeared, filtered, washed with cold absolute ethanol three times, and recrystallized with hot ethanol to obtain 3.21 g of yellow needle-shaped Schiff base crystals, with a yield of 75.4%.
[0039] H NMR spectrum 1 H-NMR (400MHz, CDCl 3 , TMS): δ=2.02 (s, 6H, CH 3 ), 2.22(s,3H,C(NAr)CH 3 ), 2.76(s, 3H, C(O)CH3 ), 6.89-7.10(m, 3H Ar-H), 7.96(m, 1H, Py-H), 8.10(d, 1H, Py-H), 8.56(d, 1H, Py-H).
[0040] Elemental Analysis C 17 h 18 N 2 O (%): calcd. C 76.67, H 6.81, N 10.53; found C 76.45, H 6.54, N 10.41.
Embodiment 2
[0041] The preparation of embodiment 2 Schiff base
[0042]
[0043] 2,6-diisopropylaniline (2.83g, 16.0mmol) was used to replace 2,6-dimethylaniline, and other operations were the same as in Example 1 to obtain 4.25g of yellow needle-shaped Schiff base crystals, with a yield of 82.5% .
[0044] H NMR spectrum 1 H-NMRH NMR (400MHz, CDCl 3 , TMS): δ=1.16-1.17(m, 12H, CH(CH 3 ) 4 ), 2.28(s,3H,C(NAr)CH 3 ), 2.74(sept, 2H, CH(CH 3 ) 2 ), 2.81(s, 3H, C(O)CH 3 ), 7.08-7.22(m, 3H, Ar-H), 7.96(t, 1H, Py-H), 8.16(d, 1H, Py-H), 8.56(d, 1H, Py-H).
[0045] Elemental Analysis: C 21 h 26 N 2 O (%): calcd. C 78.22, H 8.13, N 8.69; found C 78.39, H 8.17, N 8.84.
Embodiment 3
[0046] Example 3 Preparation of bridged pyridinediimine compound
[0047] Using the Schiff base (1.5g, 5.63mmol) obtained in Example 1 and two (3,5-dimethyl-4-aminophenyl) methane (0.72g, 2.81mmol), p-toluenesulfonic acid as a catalyst ( 0.05g), heated and stirred, separated into water and refluxed for 15h, removed most of the solvent, cooled and precipitated a yellow precipitate, filtered, and washed with ethanol several times to obtain the bridged pyridinediimine compound L 1 1.75 g, yield 83.0%.
[0048] ESI-MS: m / z=751.5[M + ].
[0049] H NMR spectrum 1 HNMR (400MHz, DCCl 3 , TMS): δ=2.04(m, 24H, Ar-Me), 2.27(m, 12H, ArN=CMe), 3.88(s, 2H, -CH 2 -), 6.95-7.09(m, 10H, Ar-H), 7.92(t, 2H, Py-H p ), 8.50 (d, 4H, Py-H m ).
[0050] Elemental Analysis: C 51 h 54 N 6 (%): Calcd.C 81.56.H 7.25.N 11.19; Found: C81.48.H 7.19.N 11.25
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com