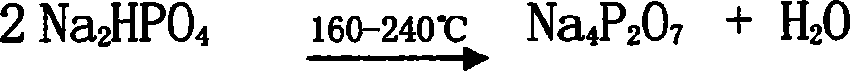Technique for preparing sodium pyrophosphate from wet method phosphoric acid
A sodium pyrophosphate, preparation process technology, applied in the direction of phosphorus compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of difficult source of raw materials, limited application range of sodium hexametaphosphate, high product cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
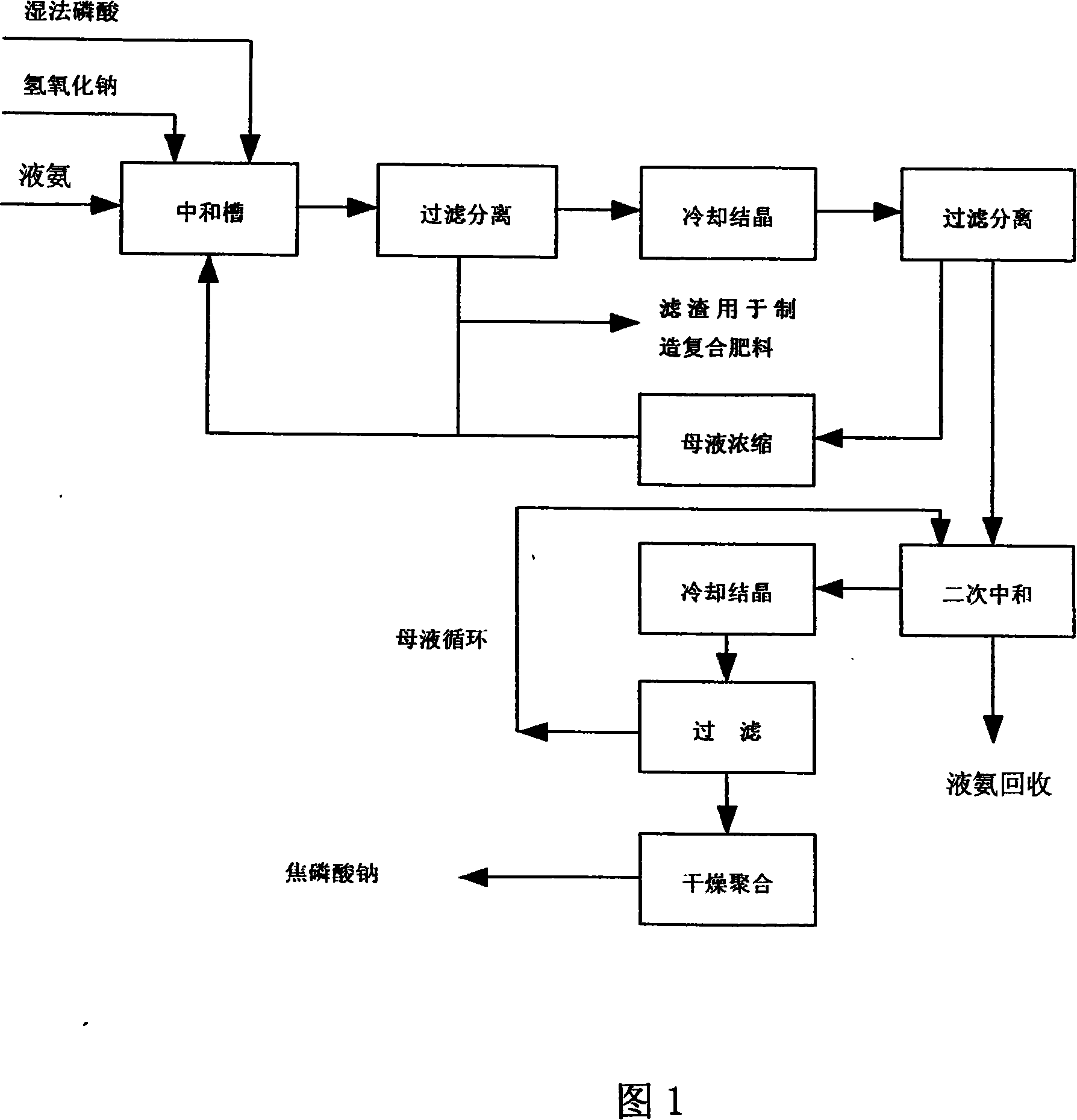
[0032] Referring to Fig. 1, the steps of this process are:
[0033] 1. Add sulfuric acid to phosphate rock powder, filter after stirring, and remove slag to obtain wet-process phosphoric acid. The P of this phosphoric acid is 2 o 5 The best concentration is ≥18%, so phosphate rock with a higher grade should be selected to increase the yield of the product.
[0034] 2. Add 1000kg 18% P 2 o 5 The wet-process phosphoric acid is placed in the neutralization tank, and 109kg of sodium hydroxide with a concentration of 98% is added to neutralize the reaction with phosphoric acid. The reaction temperature is controlled at 90°C and the reaction time is 50 minutes to prepare a slurry containing sodium dihydrogen phosphate. The reaction formula as follows
[0035] h 3 PO 4 +NaOH=NaH 2 PO 4 +H 2 o
[0036] The slurry is a mixed slurry, which contains a lot of impurities, mainly SO 2 2- , Fe 2 o 3 、Al 2 o 3 , MgO and other solid phases.
[0037] 3. Filter the slurry, colle...
Embodiment 2
[0049] Difference with embodiment one is only, in the 2nd step, replaces sodium hydroxide with 133kg 98% sodium carbonate, reacts with phosphoric acid, and reaction formula is as follows
[0050] 2H 3 PO 4 +NaCO 3 ==2NaH 2 PO 4 +H 2 O+CO 2 ↑
[0051] All the other are the same as the first embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com