Vinflunine pharmaceutical composition and method of producing the same and application of the same
The technology of a composition and chunflunine powder, which is applied in the field of vinflunine pharmaceutical composition, can solve the problems of inability to obtain vinflunine salt freeze-dried preparations and poor stability of liquid medicine, and achieve good clarity, difficulty in decomposing, Improve the effect of curative effect
Inactive Publication Date: 2010-09-08
QILU PHARMA CO LTD
View PDF1 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
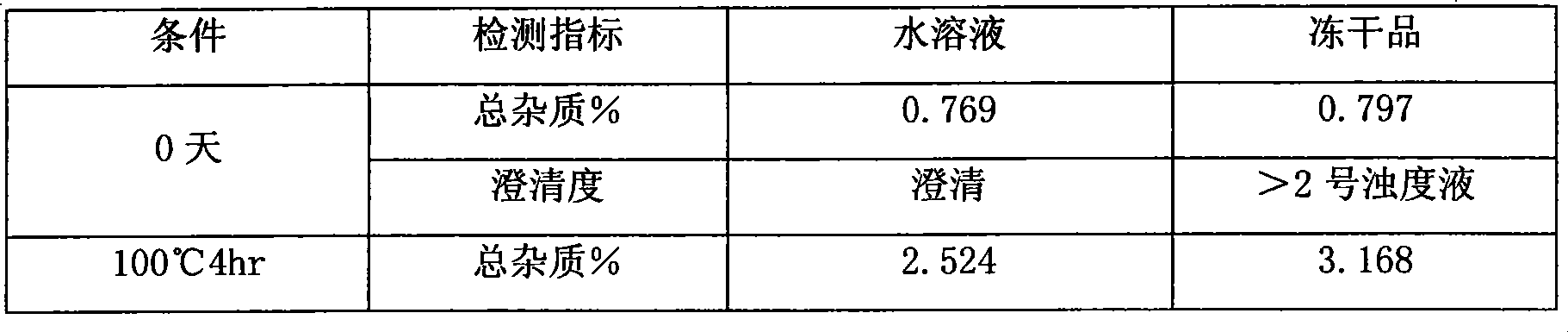
However, studies have shown that the vinflunine bitartrate powder injection sample prepared by commonly used lyophilized excipients such as mannitol at a conventional dosage has a clarity greater than No. 2 and poorer stability than the vinflunine bitartrate before freeze-drying. Obtaining an ideal and highly stable freeze-dried formulation of vinflunine salt
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
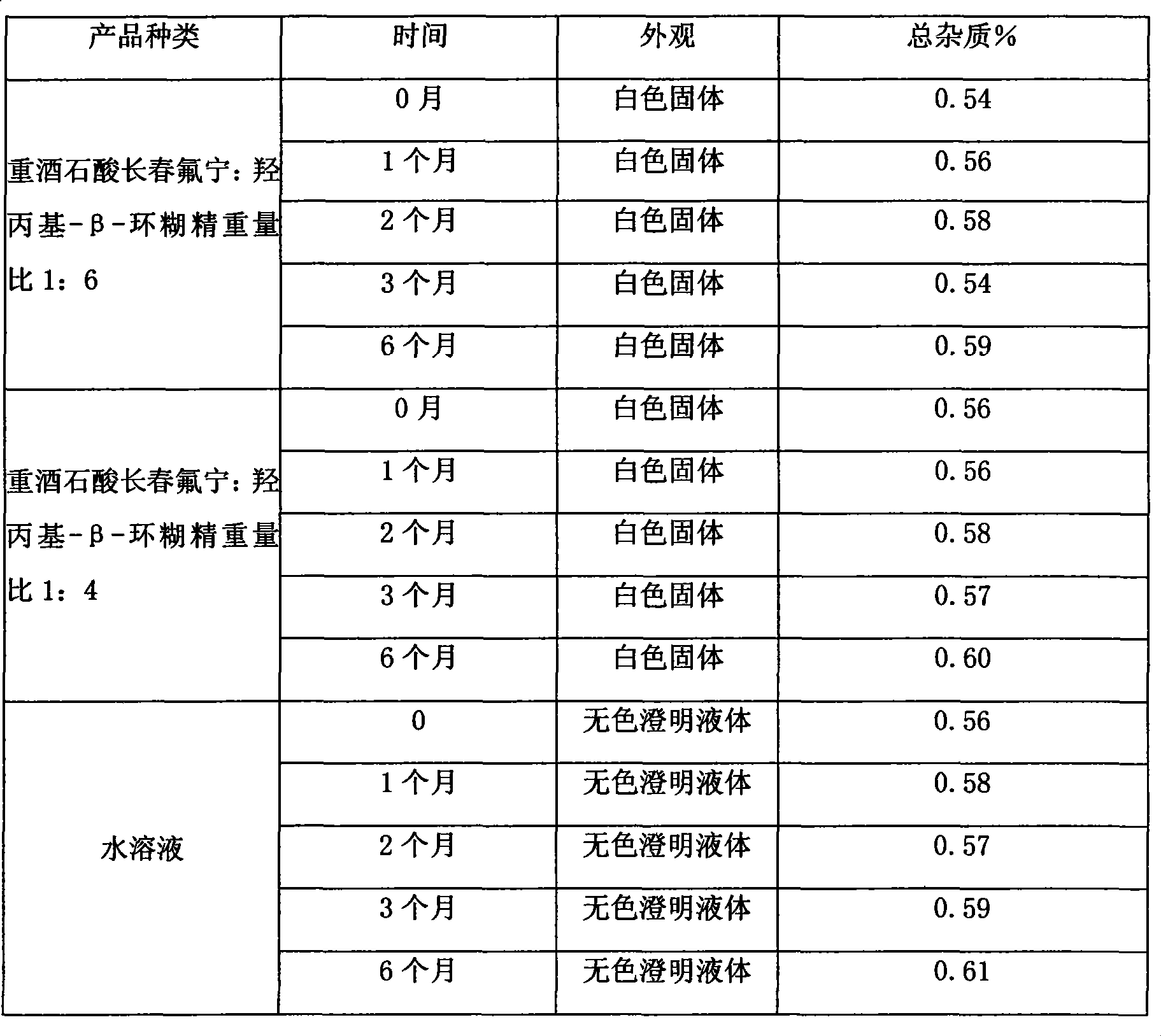
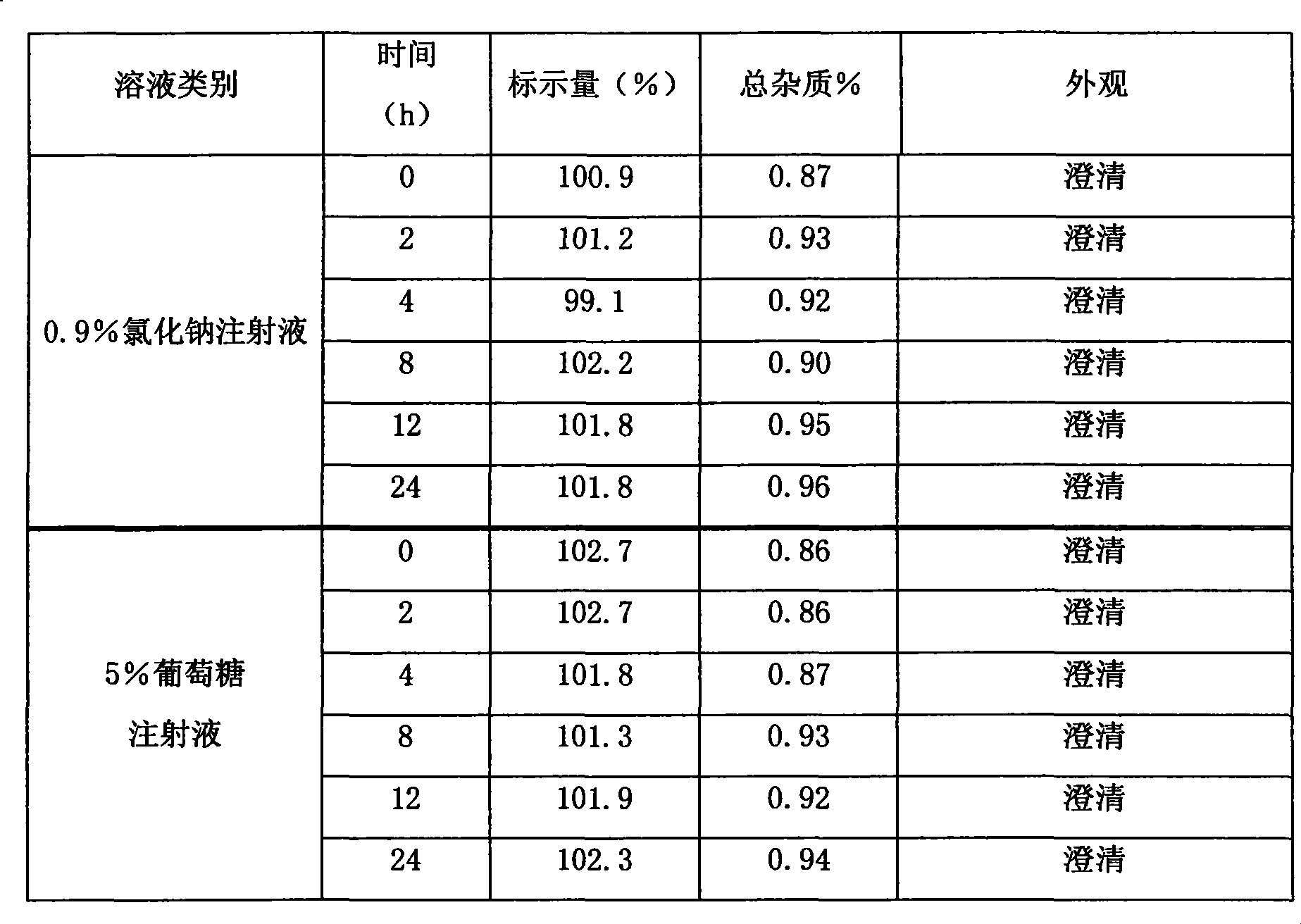
The invention relates to a pharmaceutical composition of Vinflunine, which comprises Vinflunine as the active constituent, pharmaceutically acceptable salts and cyclodextrin, wherein each 1 part of Vinflunine and its pharmaceutically acceptable salt are mixed with a maximum of 0. 5-100 parts by weight ratio of cyclodextrin, and freeze dried injection is prepared. The invention also relates to theprocess for preparing the composition, and its use in preparing non-stomach administering pharmaceutical products for the treatment of cancer.
Description
technical field The invention relates to a vinflunine pharmaceutical composition, in particular to a vinflunine freeze-dried powder injection pharmaceutical composition for parenteral administration, a preparation method thereof, and a pharmaceutical application thereof for treating cancer, belonging to the technical field of medicine . Background technique Vinflunine was originally the latest vinblastine drug screened by French company Pierre Fabre. It is derived from vinorelbine. By inhibiting tubulin polymerization, cell division is stopped in mid-mitosis. It is a cycle-specific anticancer drug. . It can be used in the treatment of non-small cell lung cancer, bladder cancer, breast cancer, mesothelioma, melanoma, kidney cancer and other tumors. It is reported in the literature that the pharmaceutically acceptable salts of vinflunine are in the form of bitartrate, sulfate and the like. The raw material of vinflunine bitartrate is very unstable and must be stored under ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K31/475A61K9/19A61K47/40A61P35/00
Inventor 徐先艳杨清敏刘宝明张明会王晶翼
Owner QILU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com