Applications of oral pharmaceutical composition to preparation of medicament for preventing or treating kidney diseases
A kidney disease and composition technology, applied in the field of medicine, can solve the problems of unacceptable, repeated injections, subcutaneous bleeding and the like for patients, and achieve the effects of convenient taking, reducing the dosage and alleviating the pain of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
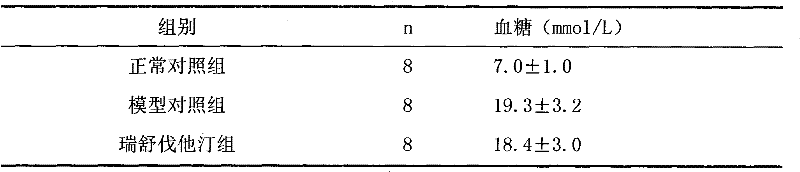
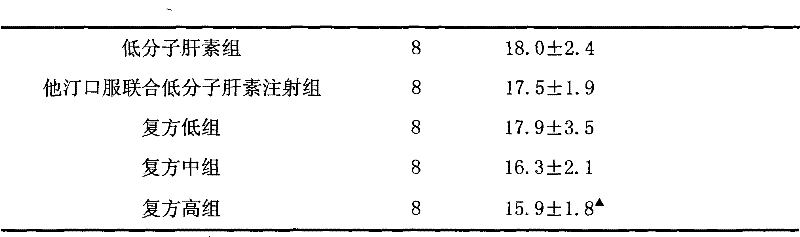
[0033] Example 1 Effect of Rosuvastatin and Low Molecular Heparin Compound on Diabetic Nephropathy in Rats
[0034] 1 Establishment of animal model
[0035] 90 male SD rats with a body weight of (250±20) g were selected. After one week of adaptive feeding, all rats were fasted for 12 hours, and streptozotocin (STZ) was intraperitoneally injected once at 65 mg / kg. Before STZ is used, prepare 2% STZ injection with sodium citrate buffer (0.1mol / L, pH4.5), and use it up within 10 minutes. All rats were housed in separate cages in the same animal room and fed with a standard diet. Feed continuously for 2 weeks, measure blood sugar, urine sugar, 24h urine microalbumin and urine β 2 Microglobulin. The selection criteria of rats with diabetic nephropathy (DN) need to meet the following three conditions at the same time: rat blood glucose higher than 16.7mmol / L, urine output more than doubled and urine albumin positive.
[0036] 2 Grouping and administration
[0037] Modeled DN r...
Embodiment 2
[0072] Example 2 Intervention Effect of Rosuvastatin and Heparin Compound on Early Kidney Damage Caused by Hypertension
[0073] 1. Grouping and administration of experimental animals
[0074] 48 male spontaneously hypertensive rats (SHR) of SPF grade, 12 weeks old, weighing 280-300 g, were provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences. 4 rats in a cage, fed with standard feed, free to eat and drink, in a constant temperature and quiet environment of 24 ± 2 degrees Celsius. At the same time, another 8 Wistar-Kyoto (WKY) of the same age were set as the normal control group. The hypertensive rats (SHR) were randomly divided into a model group and an administration group, with 8 rats in each group. All groups were intragastrically administered. The specific grouping and administration are as follows:
[0075] Normal control group: the same volume of purified water;
[0076] Model control group: the same volume of sodium carboxymethyl ...
Embodiment 3
[0103] Example 3 Effect of Rosuvastatin and Low Molecular Heparin Compound on Hypertensive Nephropathy
[0104] 1. Establishment of animal model of hypertensive nephropathy
[0105] 48 male spontaneously hypertensive rats (SHR) of SPF grade, 12 weeks old, weighing 280-300 g, were provided by the Shanghai Experimental Animal Center of the Chinese Academy of Sciences. Add 50 mg / L of nitro-L-arginine methyl ester (L-NAME) to the drinking water of all SHR rats. And replace the newly configured water bottle containing L-NAME every Monday, Wednesday, and Friday. On 0d, 14d, and 28d after the start of the experiment, 24h urine was collected from the rats, and the urine protein content was determined. Rat urinary albuminuria "++" was used as a sign of severe hypertensive nephropathy in hypertensive rats (SHR), which was used as a selection criterion for successful modeling.
[0106] 2. Grouping and administration of hypertensive nephropathy rats
[0107] Preferably, 40 rats with h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com