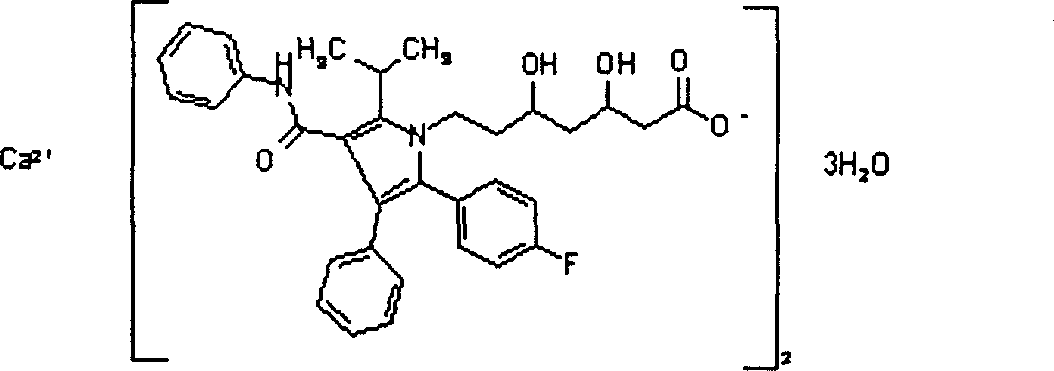
Composition for treating hyperlipemia
A technology for hyperlipidemia and composition, applied in the field of composition containing niacin and atorvastatin calcium and pharmaceutical excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0024] a. Niacin 600g
[0025] Blank ball core 250g
[0026] 7% PVP solution (solvent is 90% ethanol) 200g
[0027] Preparation process: Pass niacin through a 120-mesh sieve, weigh the prescribed amount, and pour it into the lower hopper. Turn on the granulation coating machine, the air inlet pressure is 0.5bar, the air inlet temperature is 30°C, the spray gun pressure (CYL) is 3bar, the atomization pressure (CAP1) is 0.8bar, pour blank ball cores, granulate, the feeding speed is 4rpm, the creeping Pump 12%, turntable speed 145rpm, spray 7% PVP solution (solvent is 90% ethanol). After granulation is completed, dry at 50°C and discharge.
[0028] b. Atorvastatin Calcium 5g
[0029] Blank ball core 30g
[0030] 7% PVP solution (solvent is 90% ethanol) 30g
[0031]Preparation process: pass atorvastatin calcium through a 120-mesh sieve, weigh the prescription amount, and pour it into the lower hopper. Start the granulation coating machine, the in...
example 2
[0034] a. Niacin 600g
[0035] Lactose 30g
[0036] Sodium carboxymethyl starch 30g
[0037] Microcrystalline Cellulose 18g
[0038] 6% PVP in absolute ethanol 100g
[0040] Preparation process: Niacin is passed through a 100-mesh sieve, lactose, sodium carboxymethyl starch, and microcrystalline cellulose are passed through a 80-mesh sieve, and the prescribed amount of nicotinic acid is weighed and mixed with lactose, sodium carboxymethyl starch, and microcrystalline cellulose, Add an appropriate amount of 6% PVP absolute ethanol solution to granulate, dry at 60°C, sieve the dry granules with a 16-mesh sieve, and add the prescribed amount of magnesium stearate to the dry granules.
[0041] b. Atorvastatin calcium 10g
[0042] Hydroxypropyl Cellulose 15g
[0043] 10g pregelatinized starch
[0044] 6% PVP in absolute ethanol solution 30g
[0045] Glyceryl Behenate 1g
[0046] P...
example 3
[0049] a. Niacin 600g
[0050] Lactose 30g
[0051] Sodium carboxymethyl starch 30g
[0052] Microcrystalline Cellulose 18g
[0053] 6% PVP in absolute ethanol 100g
[0055] Preparation process: Niacin is passed through a 100-mesh sieve, lactose, sodium carboxymethyl starch, and microcrystalline cellulose are passed through a 80-mesh sieve, and the prescribed amount of nicotinic acid is weighed and mixed with lactose, sodium carboxymethyl starch, and microcrystalline cellulose, Add an appropriate amount of 6% PVP absolute ethanol solution to granulate, dry at 60°C, sieve the dry granules with a 16-mesh sieve, and add the prescribed amount of magnesium stearate to the dry granules.
[0056] b. Atorvastatin calcium 20g
[0057] Hydroxypropyl Cellulose 30g
[0058] 20g pregelatinized starch
[0059] 6% PVP in absolute ethanol solution 50g
[0060] Glyceryl Behenate 2g
[0061] P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com