Thienopyridine derivatives and use thereof as hsp90 modulators
A technology of derivatives and compounds, applied in the field of new compounds, can solve problems such as incorrectly regulated molecular and physiological functions, loss of wild-type functions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
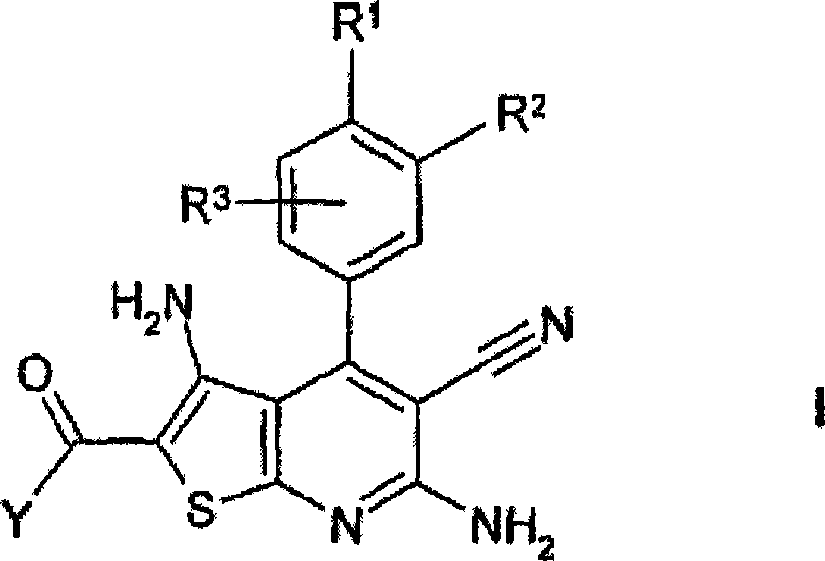
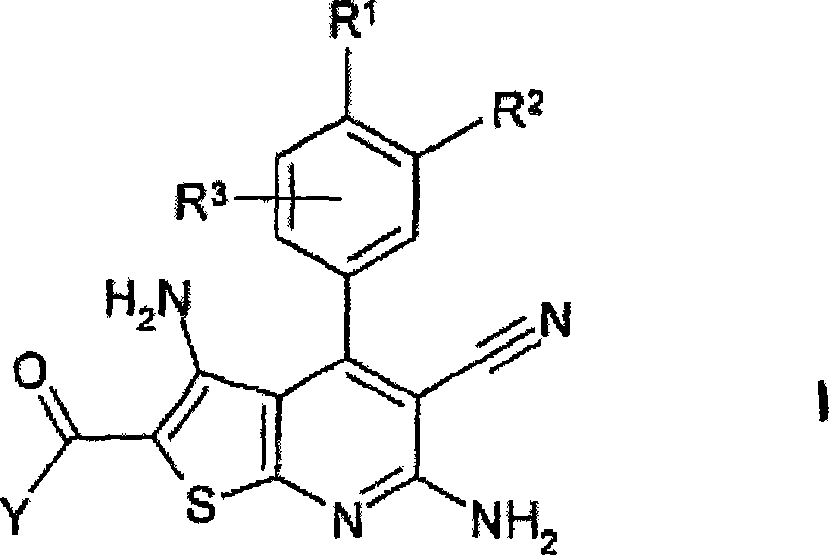
[0364] Preparation of 2-aminocarbonyl-3,6-diamino-5-cyano-4-(3,4-dimethoxyphenyl)thieno[2,3-b]pyridine ("A1"):
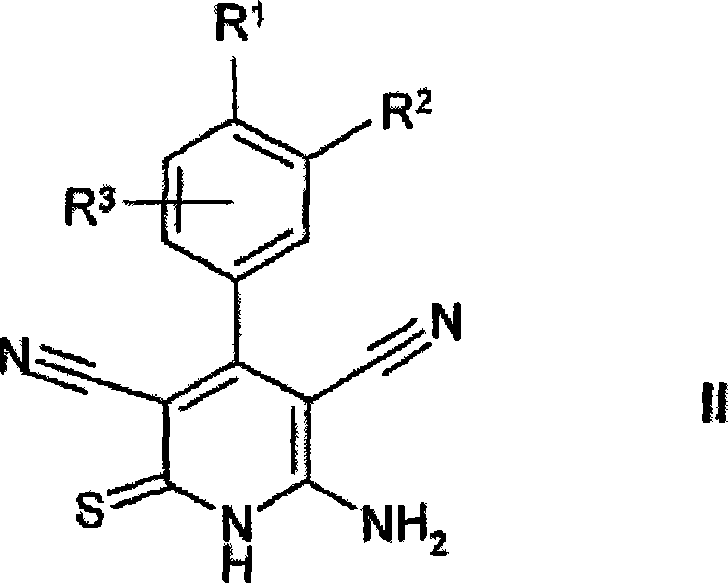
[0365] 1.1 Add 12.1 g of cyanothioacetamide to 10 g of 3,4-dimethoxybenzaldehyde in 100 mL of ethanol solution. Then 10 mL of 4-methylmorpholine were added dropwise and the mixture was stirred at room temperature for a further 16 hours. The mixture was then refluxed for 4 hours. The mixture was adjusted to pH 5.0 with 10% HCl and stirred at room temperature for an additional 16 hours. The precipitated material was separated, washed with ethanol and n-heptane and dried to give 9.2 g of 6-amino-3,5-dicyano-4-(3,4-dimethoxyphenyl)-2-thio-1 , 2-dihydropyridine ("1")
[0366]
[0367] 1.2 Add 255 μL of 47% KOH aqueous solution to 1 g of “1” in 5 mL of DMF. Then 300 mg of 2-chloroacetamide were added and the mixture was stirred for a further 1 hour at room temperature. Another 255 μL of 47% aqueous KOH was added and the mixture was stirred at room temperature for ...
Embodiment 2
[0388] 2-aminocarbonyl-3,6-diamino-5-cyano-4-[3-(5-ethoxycarbonylpentyloxy)-4-methoxyphenyl]thieno[2,3-b Preparation of ]pyridine ("A4"):
[0389]
[0390] 2.1 A mixture of 100 mg "A3", 60 μL ethyl bromohexanoate, 100 mg potassium carbonate and 1 mL DMF was stirred at 50° C. for 4 hours. The whole mixture was added to 20 mL of water, the precipitated material was separated and dried to give 129 mg of "A4", R f 1.569, MW 498.6.
[0391] The following compounds were obtained in a similar manner:
[0392] 2-aminocarbonyl-3,6-diamino-5-cyano-4-[3-(4-ethoxycarbonylbutoxy)-4-methoxyphenyl]thieno[2,3-b ]pyridine ("A7"), R f 1.450, MW 484.5.
[0393] Ester hydrolysis of "A7" in NaOH / methanol affords compound
[0394] 2-aminocarbonyl-3,6-diamino-5-cyano-4-[3-(4-carboxybutoxy)-4-methoxyphenyl]thieno[2,3-b]pyridine ( "A7a"), R f 1.531, MW 456.5.
[0395] Ester hydrolysis of "A4" in NaOH / methanol affords compound
[0396] 2-aminocarbonyl-3,6-diamino-5-cyano-4-[3-(5-carboxy...
Embodiment 3
[0402] Similar to Example 1, "1" was reacted with methyl chloroacetate to obtain the compound 2-methoxycarbonyl-3,6-diamino-5-cyano-4-(3,4-dimethoxybenzene base) thieno[2,3-b]pyridine ("A8bis").
[0403] The following compounds were obtained in a similar manner:
[0404] 2-ethoxycarbonyl-3,6-diamino-5-cyano-4-(3,4-dimethoxyphenyl)thieno[2,3-b]pyridine (“A9”),
[0405] 2-methoxycarbonyl-3,6-diamino-5-cyano-4-(3-hydroxy-4-methoxyphenyl)thieno[2,3-b]pyridine (“A8d”),
[0406] 2-methoxycarbonyl-3,6-diamino-5-cyano-4-(3-hydroxyphenyl)thieno[2,3-b]pyridine (“A8e”),
[0407] 2-methoxycarbonyl-3,6-diamino-5-cyano-4-(3-hydroxy-4-trifluoromethoxyphenyl)thieno[2,3-b]pyridine (“A8f” ),
[0408] 2-methoxycarbonyl-3,6-diamino-5-cyano-4-(3-hydroxy-4-methylsulfanylphenyl)thieno[2,3-b]pyridine (“A8g” ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com