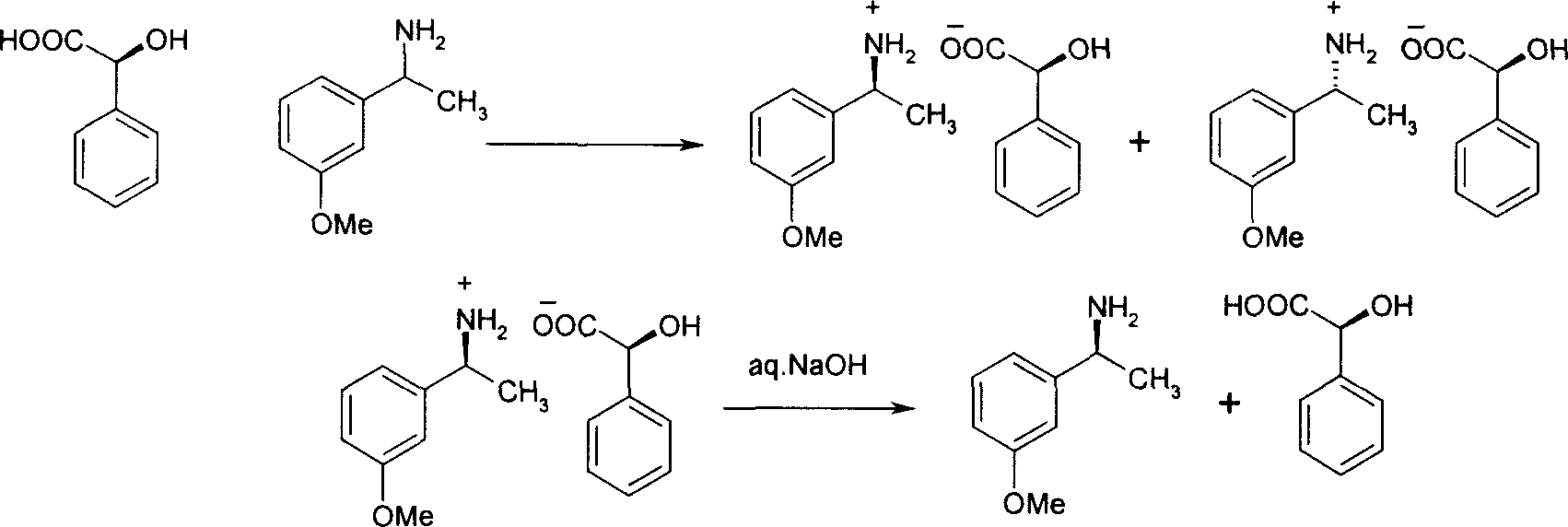
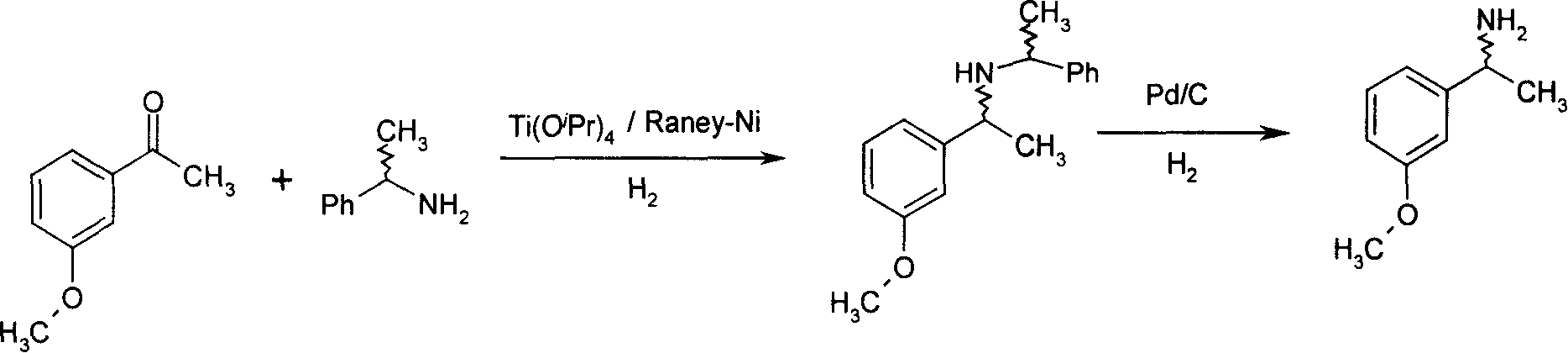
Method for preparing optical active 1-(3-methoxy phenyl) ethylamine
A technology of methoxyphenyl and methoxyacetophenone, which is applied in the field of medicinal chemistry, can solve the problems of unsatisfactory effect and low yield, and achieve the effects of mild conditions, high reaction yield and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Put 6g (0.04mol) of m-methoxyacetophenone, 4.84g (0.04mol) of S-phenethylamine and 11.36g (0.04mol) of tetraisopropyl titanate into the hydrogenation kettle, add 100ml methanol and Raney-Ni 1.8 g, react at an internal temperature of 80C and a pressure of 3 atm until the pressure does not drop, filter the discharge to remove Raney-Ni, add 40ml of 1N sodium hydroxide to the filtrate and recover methanol, extract the residue with 50ml of ethyl acetate, recover ethyl acetate and add 50ml Methanol and 10% Pd / C 1.2g, debenzylation at 60°C and 3 atm, filter off the Pd / C catalyst after the reaction is complete, collect the target product S-1-(3-methoxyphenyl)ethylamine after recovering the solvent 4.53 g, yield 75%. 1 HNMR (CDCl 3 )δ: 1.36 (d, 2H,), 3.80 (S, 3H), 4.05 (q, 1H,), 6.76~7.22 (m, 4H); ESI (m / z): 152 (M+1); (C2, MeOH).
Embodiment 2
[0027] Put 12g (0.08mol) of m-methoxyacetophenone, 11.62g (0.096mol) of S-phenethylamine and 36.48g (0.16mol) of tetraethyl titanate into the hydrogenation kettle, add 200m ethyl acetate and Raney-Ni7 .2g, react at an internal temperature of 60°C and a pressure of 5 atm until the pressure does not drop, filter the discharge to remove Raney-Ni, add 80ml of 1N sodium hydroxide to the filtrate and separate the liquid, add 200ml of ethanol and 5% Pd / C4 after recovering ethyl acetate .8g, debenzylation at 60°C and 3atm. After the reaction was complete, the Pd / C catalyst was filtered off. After recovering the solvent, 9.66g of the target product S-1-(3-methoxyphenyl)ethylamine was collected, with a yield of 80%.
Embodiment 3
[0029] Put 6g (0.04mol) of m-methoxyacetophenone, 6.3g (0.052mol) of R-phenethylamine and 68g (0.2mol) of tetrabutyl titanate into the hydrogenation kettle, add 100ml of tetrahydrofuran and 6g of Raney-Ni, React at a temperature of 70°C and a pressure of 10 atm until the pressure does not drop, filter the discharge to remove Raney-Ni, add 200ml of 1N sodium hydroxide to the filtrate and recover tetrahydrofuran, extract the residue with 50ml of ethyl acetate, add 50ml of methanol and 10 %Pd / C 1.2g, debenzylation at 60°C and 3 atm, filter off the Pd / C catalyst after the reaction is complete, collect the target product R-1-(3-methoxyphenyl)ethylamine 4.59g after recovering the solvent, and obtain rate of 76%.
[0030] 1 HNMR (CDCl 3 )δ: 1.37 (d, 2H,), 3.80 (S, 3H), 4.08 (q, 1H,), 6.76~7.22 (m, 4H); ESI (m / z): 152 (M+1); (C2, MeOH).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap