N-hydroxyamide derivatives and use thereof
A technology of hydroxyamides and derivatives, which is applied in the field of N-hydroxyamide derivatives and their applications, and can solve the problems of dose-limiting side effects and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
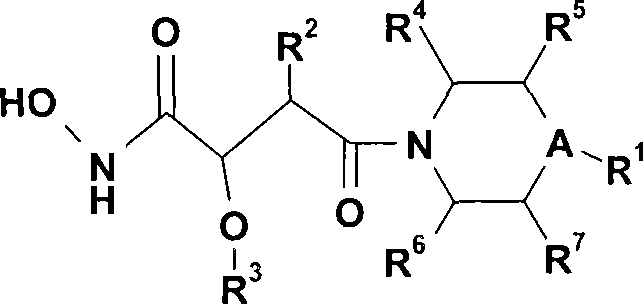
[0154] In another specific embodiment, the present invention provides the preparation method of N-hydroxyl amide derivative of the present invention, comprises making formula (IV) compound and H 2 NO-R 8 Steps in the derivative reaction:
[0155]
[0156] where A, R 1 , R 2 , R 4 , R 5 , R 6 and R 7 As defined above, R 8 selected from H and a protecting group such as tert-butyl, benzyl, trialkylsilyl, tetrahydropyranyl.
[0157] In yet another specific embodiment, the present invention provides the preparation method of N-hydroxyamide derivatives of the present invention, optionally further comprising a deprotection step (when R 8 When not H, remove R 8 ).
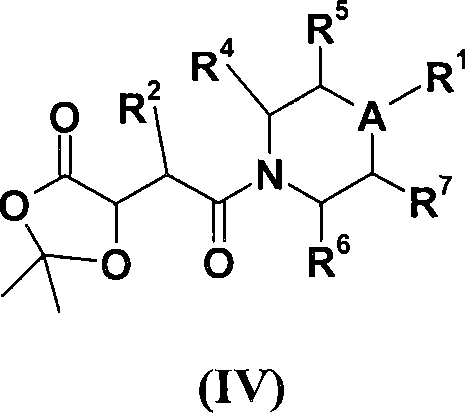
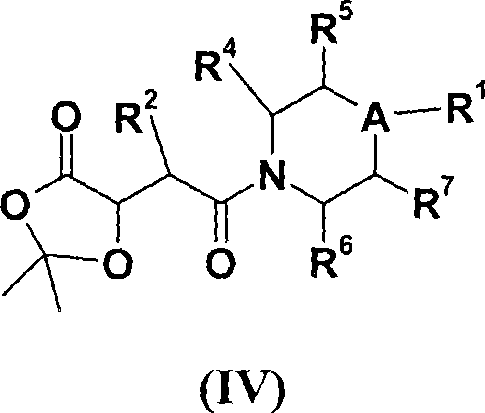
[0158] In another specific embodiment, the present invention provides compounds represented by formula (IV):
[0159]
[0160] where A, R 1 , R 2 , R 4 , R 5 , R 6 and R 7 as defined above.
[0161] In yet another specific embodiment, the present invention provides a compound of formula (IV) selecte...
Embodiment 1
[0244] Example 1: (2R)-4-[4-(4-fluorophenyl)piperazin-1-yl]-N,2-dihydroxy-4-oxobutanamide (1)
[0245]
[0246] Step a) generates (5R)-5-{2-[4-(4-fluorophenyl)piperazin 1-yl]-2-oxoethyl}-2,2-dimethyl-1,3-di Oxolane-4-one
[0247]
[0248]HOBt (2.97 g; 22.0 mmol; 1.1 eq.) was added to [(4R)-2,2-dimethyl-5-oxo-1,3-dioxolan-4-yl]acetic acid (3.48 g; 20.0 mmol; 1.0 eq.), TEA (6.07 g; 60.0 mmol; 3.0 eq.) in DCM (60 mL), and the mixture was cooled to 0 °C. EDC (4.6 g; 24.0 mmol; 1.2 eq.) was then added and the resulting reaction mixture was stirred at 0° C. for 15 minutes. 1-(4-Fluorophenyl)piperazine dihydrochloride (5.57 g; 22.0 mmol; 1.1 eq.) was added and the resulting reaction mixture was stirred at room temperature overnight. Purification by flash chromatography (AcOEt / c-Hex: 50 / 50) afforded the title compound as a colorless oil (5.12 g, 76%). m + (ESI): 337.2. HPLC (Condition A): Rt: 2.5 min (HPLC purity: 97.4%).
[0249] Step b) generates (2R)-4-[4-(4-fluorophe...
Embodiment 2
[0251] Example 2: (2S)-4-[4-(4-fluorophenyl)piperazin-1-yl]-N,2-dihydroxy-4-oxobutanamide (2)
[0252]
[0253] Step a) generates (5S)-5-{2-[4-(4-fluorophenyl)piperazin-1-yl]-2-oxoethyl}-2,2-dimethyl-1,3- Dioxolan-4-one
[0254]
[0255] The title product was prepared following the procedure of Preparative Example 1 (step a), but starting from [(4S)-2,2-dimethyl-5-oxo-1,3-dioxolan-4-yl]acetic acid (300 mg ; 1.72 mmol; 1.0 eq.), the title compound was obtained as a white foam (350 mg, 60%). m + (ESI): 337.1. 1 H NMR (CDCl 3 , 300 MHz) δ7.12-6.83(m, 4H), 4.94(dd, J=3.0Hz, J=7.5Hz, 1H), 3.90-3.68(m, 2H), 3.70-3.57(m, 2H), 3.19-3.08(m, 4H), 3.05(dd, J=3.0Hz, J=16.6Hz, 1H), 2.85(dd, J=7.5Hz, J=16.6Hz, 1H), 1.68(s, 3H), 1.63 (s, 3H). HPLC (condition A): Rt: 2.6 min (HPLC purity: 96.9%).
[0256] Step b) generates (2S)-4-[4-(4-fluorophenyl)piperazin-1-yl]-N,2-dihydroxy-4-oxobutyramide (2)
[0257] The title product was prepared following the procedure of Preparative E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com