Naluminum fluoride base fluorating catalyst and preparation thereof
A technology based on fluorination catalyst and fluorination catalyst is applied in the field of aluminum fluoride-based fluorination catalyst and its preparation, which can solve the problems that the catalyst preparation process cannot be carried out continuously, the catalyst reproducibility cannot be guaranteed, the catalyst preparation process is complicated, and the like, To achieve the effect of shortening the preparation cycle, reducing the time of fluorination activation, and being easy to control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Dissolve chromium nitrate in water, react with precipitant ammonia water at 60°C, adjust the pH value of the reaction solution in the range of 7.5 to 8.5, make it fully precipitate under stirring conditions, filter the formed slurry, and use deionized Wash with water until neutral, then dry at 120°C for 12 hours to obtain Cr(OH) 3 .
[0031] Ammonium hexafluoroaluminate and the obtained Cr(OH) 3 Mix uniformly according to the mass ratio of 80:20, and press into tablets to form the catalyst precursor. The catalyst precursor was calcined in a muffle furnace at 450°C for 8 hours, then loaded into a tubular reactor, heated to 300°C, fed with hydrogen fluoride gas for fluorination for 1 hour, then raised to 350°C at a heating rate of 1°C / min, and continued After fluorination for 8 hours, an aluminum fluoride-based fluorination catalyst was obtained.
[0032] The specific surface area of the catalyst was measured by BET low temperature nitrogen adsorption method to be 55...
Embodiment 2
[0036] The preparation process of the catalyst is basically the same as in Example 1, except that ammonium hexafluoroaluminate is changed to ammonium tetrafluoroaluminate.
[0037] The specific surface area of the catalyst was determined by BET low temperature nitrogen adsorption method to be 45.3m 2 g -1 , the pore volume is 0.2ml·g -1 , and the proportion of pores with a diameter smaller than 2nm was 28%.
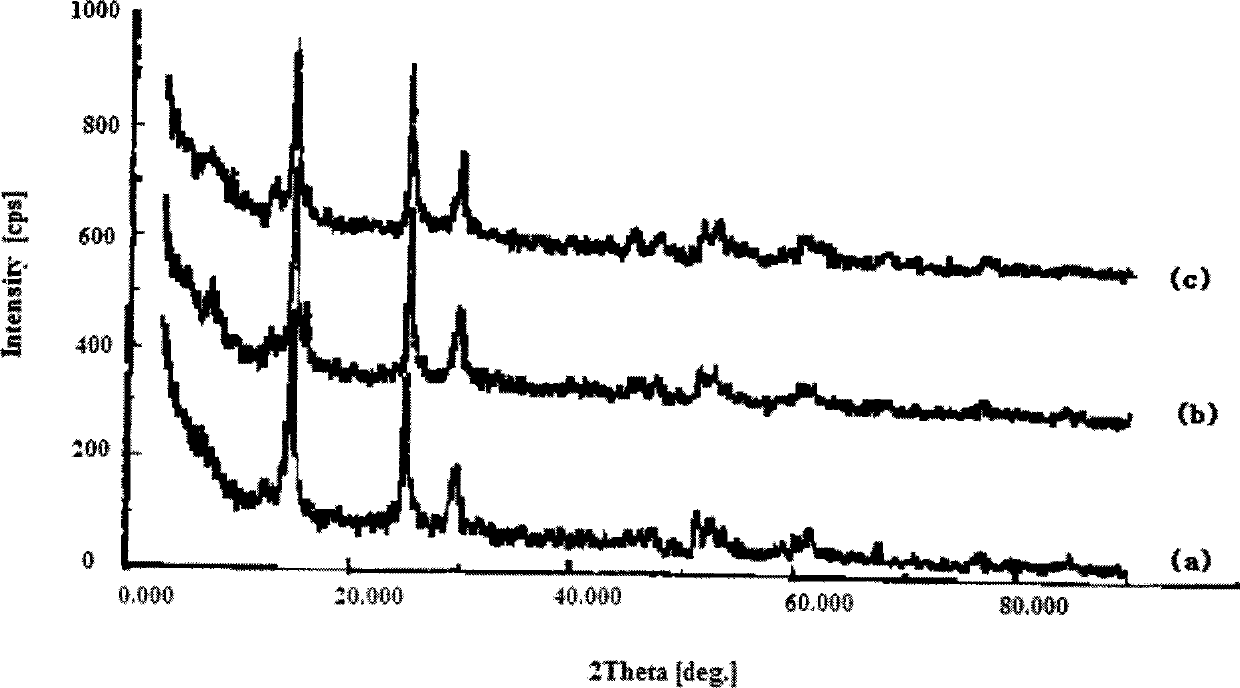
[0038] The aluminum fluoride in the calcined catalyst precursor and the fresh aluminum fluoride-based fluorination catalyst was measured by X-ray diffractometer in gamma crystal form, and chromium was in amorphous form.
[0039] In a nickel tube fixed-bed tubular reactor with an internal diameter of 38 mm, 30 ml of the aluminum fluoride-based fluorination catalyst prepared above are charged, and HF and HCFC-123 are introduced to react, and the molar ratio of HF / HCFC-123 is controlled to be 10:1, the contact time is 10 seconds, the reaction temperature is 330 ° C, aft...
Embodiment 3
[0041] The preparation process of the catalyst is basically the same as in Example 1, except that ammonium hexafluoroaluminate and Cr(OH) in the catalyst precursor 3 The mass ratio is 95:5.
[0042] The specific surface area of the catalyst was measured by BET low temperature nitrogen adsorption method to be 50.4m 2 g -1 , the pore volume is 0.18ml·g -1 , and the proportion of pores with a diameter smaller than 2nm was 33%.
[0043] The aluminum fluoride in the calcined catalyst precursor and the fresh aluminum fluoride-based fluorination catalyst was measured by X-ray diffractometer in gamma crystal form, and chromium was in amorphous form.
[0044] In a nickel tube fixed bed tubular reactor with an internal diameter of 38 mm, 30 ml of the aluminum fluoride-based fluorination catalyst prepared above are loaded into it, and HF and vinylidene chloride are introduced to react, and the mol ratio of HF / vinylidene chloride is controlled to be 8:1, the contact time is 5 second...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com