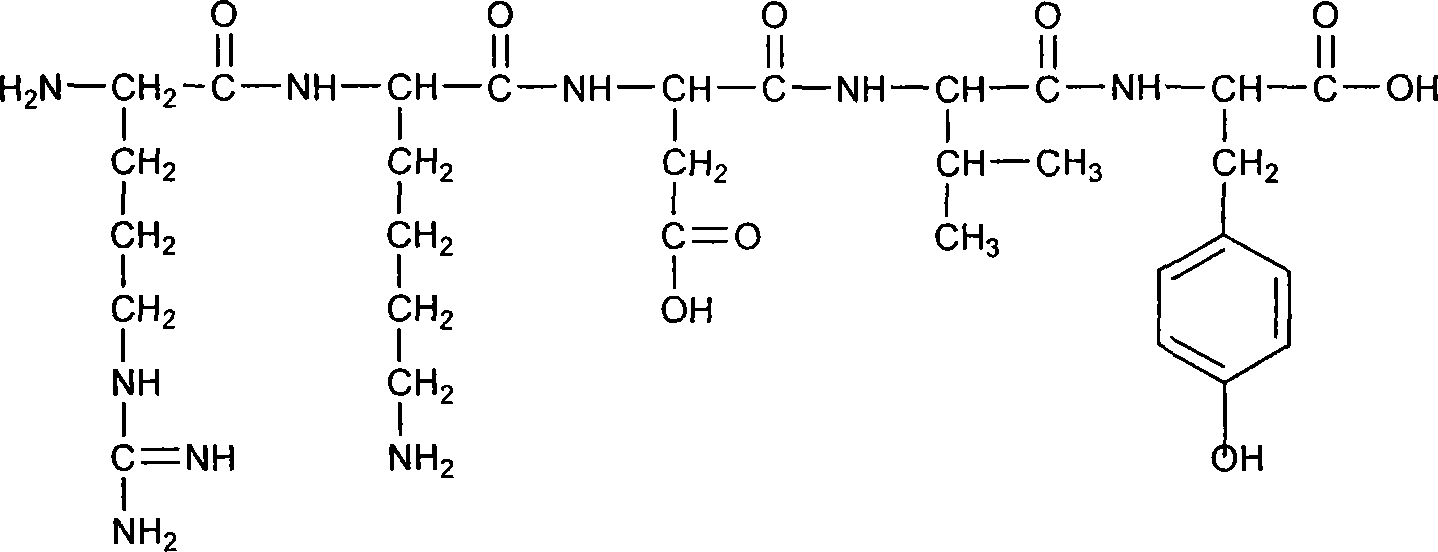
Thymus gland pentapeptide injection and uses thereof
An injection and thymus technology, which is applied in the field of medicine, can solve the problems of patients with storage conditions using denatured and ineffective medicines, and achieve the effects of eliminating the possibility of infection, solving stability problems, and reducing energy consumption and costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
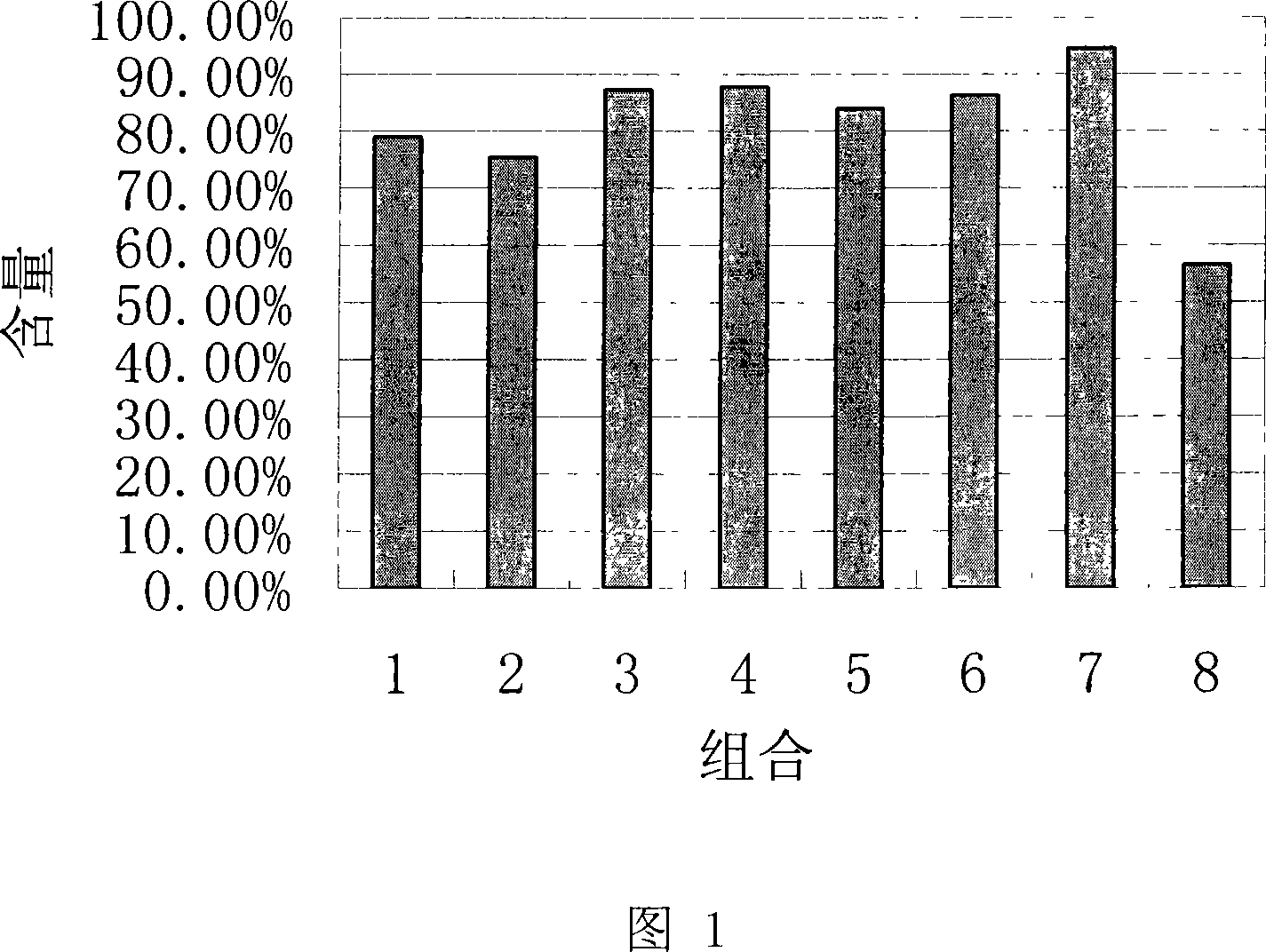
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 Thymopentin injection of the present invention
[0042] (1) Prescription (dosage per 1000 bottles of injection, specification: 1ml: 1mg thymopentin)
[0043] Thymopentin 1.0g
[0044] Mannitol 40.0g
[0045] Sodium metabisulfite 2.0g
[0046] Acetic acid and sodium acetate (3:4) 5.0g
[0047] Appropriate amount of water for injection
[0048] Full volume 1000ml
[0049] (2) Accurately weigh thymopentin and each auxiliary material respectively by prescription quantity;
[0050] (3) Add thymopentin, mannitol and sodium metabisulfite of the prescription amount into about 950ml water for injection respectively, and stir until completely dissolved;
[0051] (4) measure the pH value, adjust the pH value to 5.0 with acetic acid and sodium acetate;
[0052] (5) Supplement water for injection to 1000ml;
[0053] (6) remove the pyrogen by ultrafiltration column, and then filter and sterilize with a filter sterilized by high pressure steam;...
Embodiment 2
[0056] The preparation of embodiment 2 Thymopentin injection of the present invention
[0057] (1) Prescription (dosage per 1000 bottles of injection, specification: 1ml: 10mg)
[0058] Thymopentin 10.0g
[0059] Mannitol 75.0g
[0060] Sodium metabisulfite 2.0g
[0061] Acetic acid and sodium acetate (3:4) 5.0g
[0062] Appropriate amount of water for injection
[0063] Full volume 1000ml
[0064] (2) Accurately weigh thymopentin and each auxiliary material respectively by prescription quantity;
[0065] (3) Add thymopentin, mannitol and sodium metabisulfite of the prescription amount into about 950ml water for injection respectively, and stir until completely dissolved;
[0066] (4) measure the pH value, adjust the pH value to 6.0 with acetic acid and sodium acetate;
[0067] (5) Supplement water for injection to 1000ml;
[0068] (6) remove the pyrogen by ultrafiltration column, and then filter and sterilize with a filter sterilized by high pressure steam;
[0069...
Embodiment 3
[0071] The preparation of embodiment 3 Thymopentin injection of the present invention
[0072] (1) Prescription (dosage per 1000 bottles of injection, specification: 1ml: 5mg)
[0073] Thymopentin 5.0g
[0074] Mannitol 60.0g
[0075] Sodium metabisulfite 3.0g
[0076] Acetic acid and sodium acetate (3:7) 8.0g
[0077] Appropriate amount of water for injection
[0078] Full volume 1000ml
[0079] (2) Accurately weigh thymopentin and each auxiliary material respectively by prescription quantity;
[0080] (3) Add thymopentin, mannitol and sodium metabisulfite of the prescription amount into about 950ml water for injection respectively, and stir until completely dissolved;
[0081] (4) measure the pH value, adjust the pH value to 6.0 with acetic acid and sodium acetate;
[0082] (5) Supplement water for injection to 1000ml;
[0083] (6) remove the pyrogen by ultrafiltration column, and then filter and sterilize with a filter sterilized by high pressure steam;
[0084] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com