Medicament composition for curing diabetic cardiovascular pathological changes and method of preparing the same
A technology of diabetes and composition, applied in the field of composition for treating diabetic cardiovascular disease and its preparation field, can solve the problems of low purity of arctiside, unfavorable production quality control and improvement of drug effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
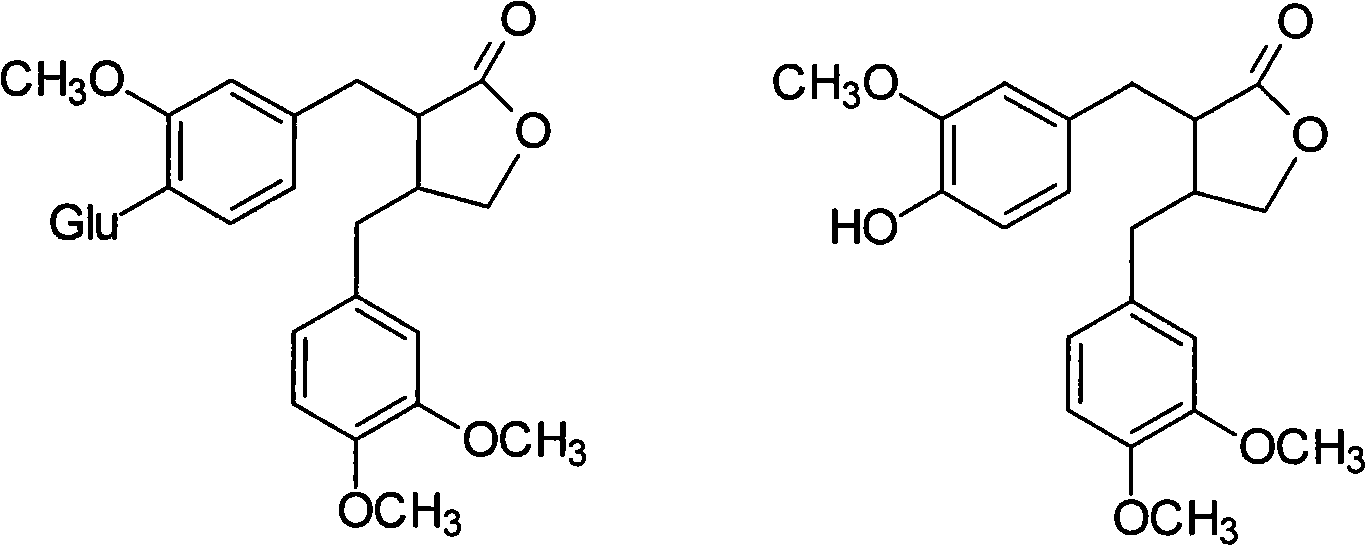
[0077] Example 1 Preparation method of arctiin and arctigenin
[0078] (1) Preparation of arctiin
[0079] ① Crude extraction of arctiin: After pulverizing the burdock seed, place it in a Soxhlet extractor and degrease it by refluxing petroleum ether at a boiling range of 60-90°C for 2 times. After degreasing, take it out and dry it, and extract it by refluxing with 60% 8 times ethanol for 2 times. 0.8h each time, the extract was concentrated under reduced pressure at 60°C to obtain a dark brown extract;
[0080] ②Refining of macroporous resin: add 15% ethanol to the dark brown extract, ultrasonically treat it for 15 minutes to make it just fully dissolve, filter and adjust to a solution with a concentration of about 5mg / mL, and pass it through the AB-8 type macroporous adsorption resin. The volume of the macroporous adsorption resin is 1 times the chemical solution, the flow rate is controlled to 1.5BV / h, the column diameter to height ratio is controlled to 1:5, the volume of the...
Example Embodiment
[0085] Example 2 Preparation method of arctiin and arctigenin
[0086] (1) Preparation of arctiin
[0087] ① Crude extraction of arctiin: After pulverizing the burdock seed, place it in a Soxhlet extractor and degrease it with petroleum ether at a boiling range of 60-90℃ for 4 times. After degreasing, take it out and dry it, and extract it with 80% 8 times ethanol under reflux for 4 times. Every 2h, the extract was concentrated under reduced pressure at 80°C to obtain a dark brown extract;
[0088] ②Refining of macroporous resin: add dark brown extract with 35% ethanol, ultrasonic treatment for 35 minutes to make it just fully dissolve, filter and adjust to a solution with a concentration of about 7mg / mL, pass it through AB-8 type macroporous adsorption resin, The volume of the macroporous adsorption resin is twice that of the chemical solution, the flow rate is controlled at 2.5BV / h, the column diameter-to-height ratio is controlled at 1:5, the volume of the resin is washed 3 tim...
Example Embodiment
[0093] Example 3 Preparation method of arctiin and arctigenin
[0094] (1) Preparation of arctiin
[0095] ① Crude extraction of arctiin: After pulverizing the burdock seed, place it in a Soxhlet extractor and degrease it by refluxing petroleum ether at a boiling range of 60-90℃ for 3 times. After degreasing, take it out and dry it, and extract it with 70% 8 times ethanol reflux for 3 times. For 1 hour each time, the extract was concentrated under reduced pressure at 70°C to obtain a dark brown extract;
[0096] ②Refining of macroporous resin: add 20% ethanol to the dark brown extract, ultrasonically treat it for 20 minutes to make it just fully dissolve, filter and adjust to a solution with a concentration of about 6mg / mL, and pass it through the AB-8 type macroporous adsorption resin. The volume of the macroporous adsorption resin is 1.5 times the volume of the chemical solution, the flow rate is controlled to 2BV / h, the column diameter to height ratio is controlled to 1:5, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com