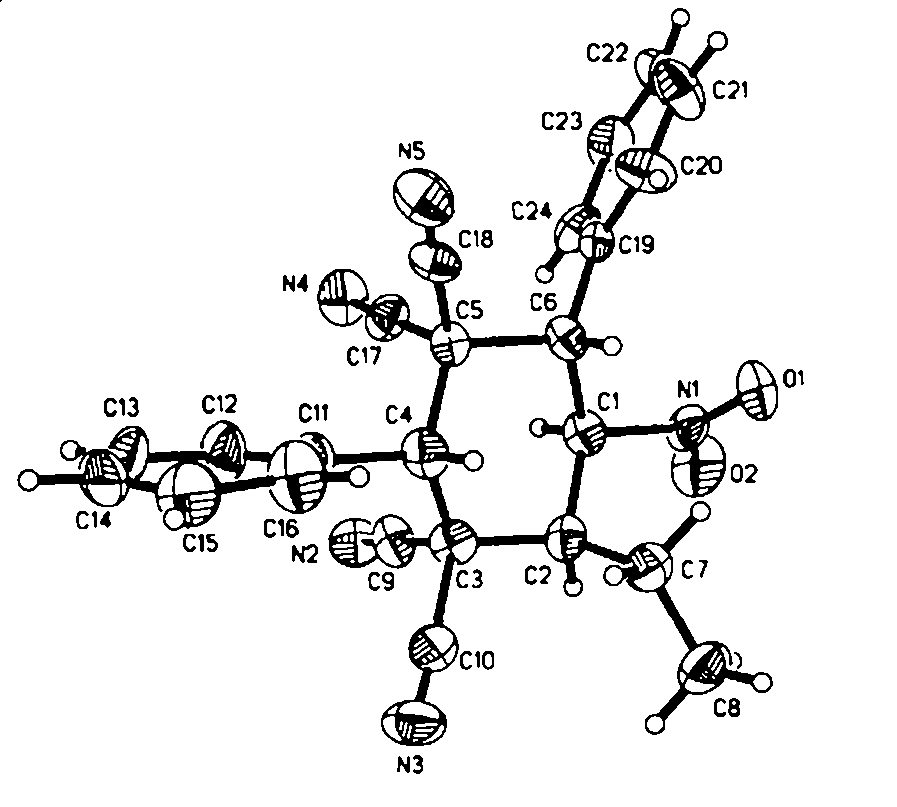
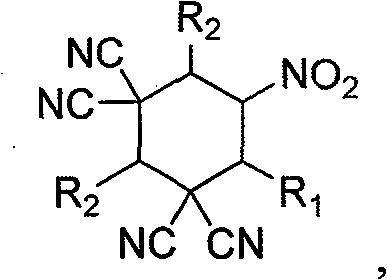
Polysubstituted cyclohexane and process for high selectively synthesizing polysubstituted cyclohexane by catalytic action of glyoxaline
A technology of cyclohexane and compound, applied in the field of synthesis of highly substituted cyclohexane, can solve the problem that the reaction is not reported in literature and the like, and achieve the effects of novel structure, high selectivity and efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] In a reaction test tube equipped with a magnetic stirring bar, compound 1a (0.15 mmol), 2a (0.30 mmol) and imidazole (0.075 mmol) were added, and then 0.3 mL of tetrahydrofuran was added, and stirred at room temperature for 24 hours. After the reaction, the product 3a was obtained by flash column chromatography, and the eluent was (petroleum ether / ethyl acetate: 8:1-4:1).
[0046] Compound 3a.mp.214-215°C; IR(CH 2 Cl 2 )ν3066, 3037, 2982, 2942, 2883, 2244, 1562, 1497, 1458, 1366, 1299, 1004, 737, 701 cm -1 ; 1 H NMR (CDCl 3 , 300 MHz, TMS): δ 1.15 (3H, t, J=7.5 Hz, CH 3 ), 1.94-2.18 (2H, m, CH 2 ), 3.30 (1H, q, J = 4.5 Hz, CH), 3.56 (1H, s, CH), 4.00 (1H, d, J = 12.0 Hz, CH), 5.80 (1H, dd, J 1 = 4.5 Hz, J 2 =12.0 Hz, CH), 7.48-7.63 (8H, m, Ar), 7.82-7.85 (2H, m, Ar). 13 C NMR (CDCl 3 , 75 MHz): δ14.9, 20.4, 42.0, 45.1, 47.6, 48.0, 48.2, 81.4, 109.8, 111.2, 111.6, 112.2, 129.2, 129.3, 129.7, 130.2, 130.3, 130.7, 131.7.MS(EI)m / z 409(M + C 24 h 19 N 5 o 2 (...
Embodiment 2
[0048] In a reaction test tube equipped with a magnetic stirring bar, add compound 1a (0.15 mmol), 2b (0.30 mmol) and imidazole (0.075 mmol), and then add 0.3 mL of tetrahydrofuran, and stir at room temperature for 24 hours. After the reaction, the product 3b was obtained by flash column chromatography, and the eluent was (petroleum ether / ethyl acetate: 8:1-4:1).
[0049] Compound 3b.mp.204-205°C; IR(CH 2 CI 2 )ν 3052, 2978, 2940, 1664, 1567, 1479, 1367, 1265, 1079, 738 cm -1 ; 1 H NMR (CDCl 3 , 300 MHz, TMS): δ 1.14 (3H, t, J=7.2 Hz, CH 3 ), 1.92-2.11 (2H, m, CH 2 ), 3.33 (1H, q, J = 4.2 Hz, CH), 3.51 (1H, s, CH), 3.95 (1H, d, J = 12.0 Hz, CH), 5.73 (1H, dd, J 1 = 4.2 Hz, J 2 =12.0 Hz, CH), 7.37 (1H, t, J=8.1 Hz, Ar), 7.48 (2H, t, J=8.1Hz, Ar), 7.63-7.67 (2H, m, Ar), 7.74-7.78 ( 1H, m, Ar), 7.87-7.92 (2H, m, Ar). 13 C NMR (CDCl 3 , 75 MHz): δ14.8, 20.3, 41.7, 44.5, 46.9, 47.3, 48.0, 81.0, 109.3, 110.9, 111.1, 111.8, 123.7, 124.1, 127.1, 131.1, 131.2, 132.2, 132.3, 1...
Embodiment 3
[0051] In a reaction test tube equipped with a magnetic stirring bar, compound 1a (0.15 mmol), 2c (0.30 mmol) and imidazole (0.075 mmol) were added, and 0.3 mL of tetrahydrofuran was added, and stirred at room temperature for 24 hours. After the reaction, the product 3c was obtained by flash column chromatography, and the eluent was (petroleum ether / ethyl acetate: 8:1-4:1).
[0052] Compound 3c.mp.211-212°C; IR(CH 2 Cl 2 )ν 3067, 2979, 2942, 1660, 1592, 1566, 1493, 1417, 1365, 1079, 1011, 830, 740 cm -1 ; 1 H NMR (CDCl 3 , 300 MHz, TMS): δ 1.15 (3H, t, J=7.2 Hz, CH 3 ), 1.93-2.12 (2H, m, CH 2 ), 3.31 (1H, q, J = 4.5 Hz, CH), 3.51 (1H, s, CH), 3.96 (1H, d, J = 11.7 Hz, CH), 5.72 (1H, dd, J 1 = 3.9 Hz, J 2 =11.7 Hz, CH), 7.39 (2H, d, J=8.7 Hz, Ar), 7.64 (2H, t, J=8.7 Hz, Ar), 7.68-7.75 (4H, m, Ar). 13 C NMR (CDCl 3 , 75 MHz): δ 14.9, 20.3, 41.7, 44.6, 47.1, 47.5, 48.1, 109.5, 111.0, 111.4, 111.9, 125.4, 126.4, 128.0, 129.1, 130.7, 133.1, 133.6. MS(EI) m / z 569 (M + +2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com