Medicine composition for treating progressive nerve degeneration disease
A pharmaceutical composition and neurodegeneration technology, applied in the direction of drug combination, pharmaceutical formula, neurological diseases, etc., can solve the problem of high risk of neurons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Granulocyte colony-stimulating factor ameliorates learning deficit in a mouse model of Alzheimer's disease
[0035] Alzheimer's-like progressive neurodegeneration was induced in mice by intraventricular injection of aggregated Aβ-type peptides (as previously described by Yan et al.)
[0036] Aggregated Aβ-type peptides were prepared from 10 mM soluble Aβ (1-42) Prepare in solution in 0.01 M phosphate buffer, pH 7.4. Aβ-type peptides were purchased from Sigma-Aldrich (St. Louis, MO). Aβ solutions were heated at 37°C for three days to form aggregated Aβ-type peptides and stored at -70°C before use. Eight-week-old C57BL / 6 male mice were anesthetized with intraperitoneal sodium pentobarbital (40 mg / kg) before injection of aggregated Aβ-type peptides. The aggregated A[beta]-type peptides were then injected stereotaxically into the dorsal hippocampus and both sides of the cerebral cortex using a 26-gauge needle attached to a Hamilton microregulation syringe (Ha...
Embodiment 2
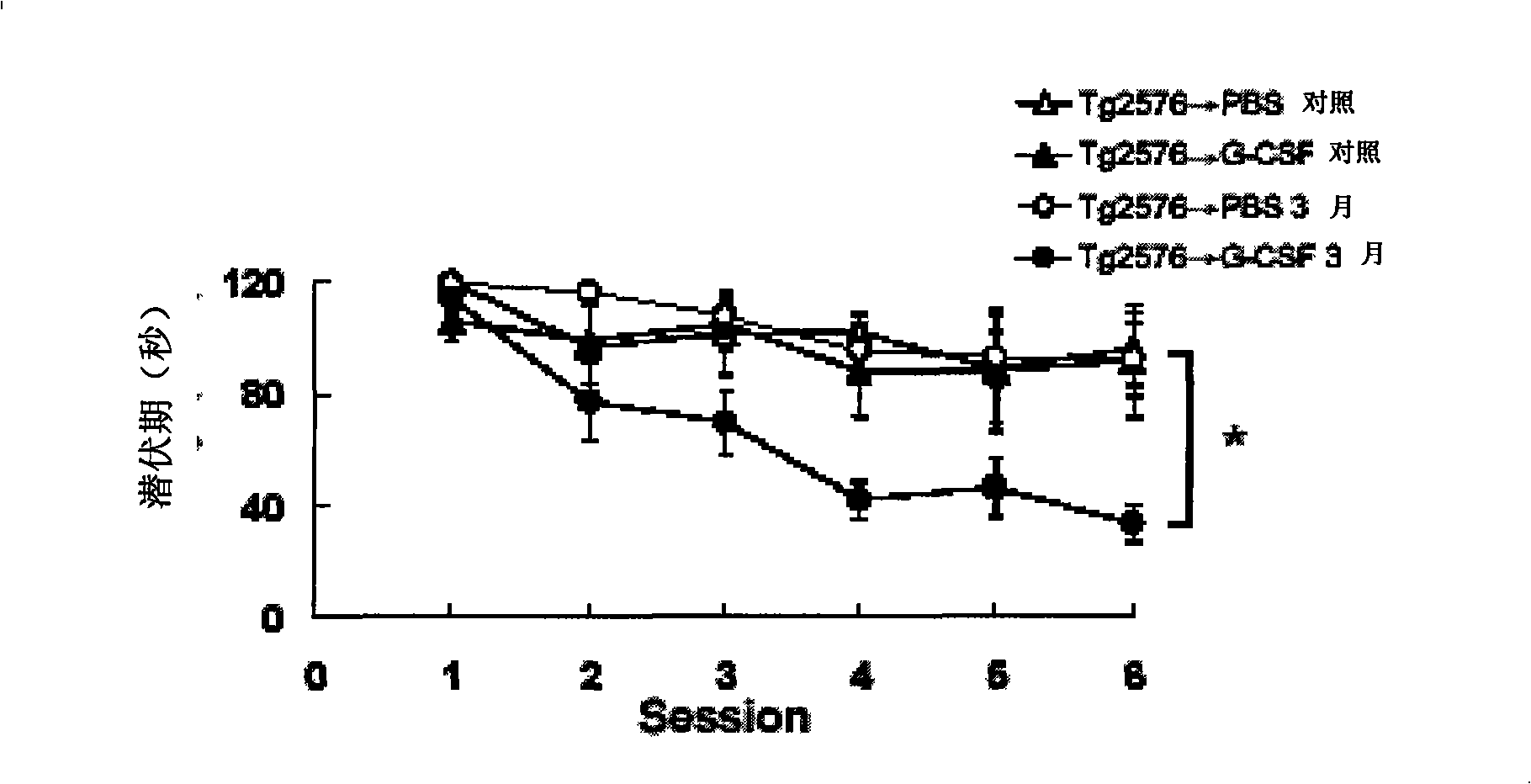
[0044] Long-lasting effects of granulocyte colony-stimulating factor
[0045] In order to test whether granulocyte colony-stimulating factor (G-CSF) can have a long-term therapeutic effect, the cognitive ability of Tg2576 mice was analyzed 3 months after treatment. To this end, 10 12-month-old mice were divided into two groups and tested with a water maze task as described above before and 3 months after subcutaneous injection of PBS and granulocyte colony-stimulating factor (G-CSF), respectively. like figure 1 As shown, the average latency of Tg2576 mice treated with PBS (3 months after Tg2576 injected with PBS) and not treated with PBS (Tg2576 administered with PBS injected control) was generally high. Clearly, however, granulocyte colony-stimulating factor-treated mice had significantly better learning / memory abilities, even 3 months after treatment (Tg2576 injected with G-CSF 3 months later vs Tg2576 administered with G-CSF injection control comparison). These data demo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com