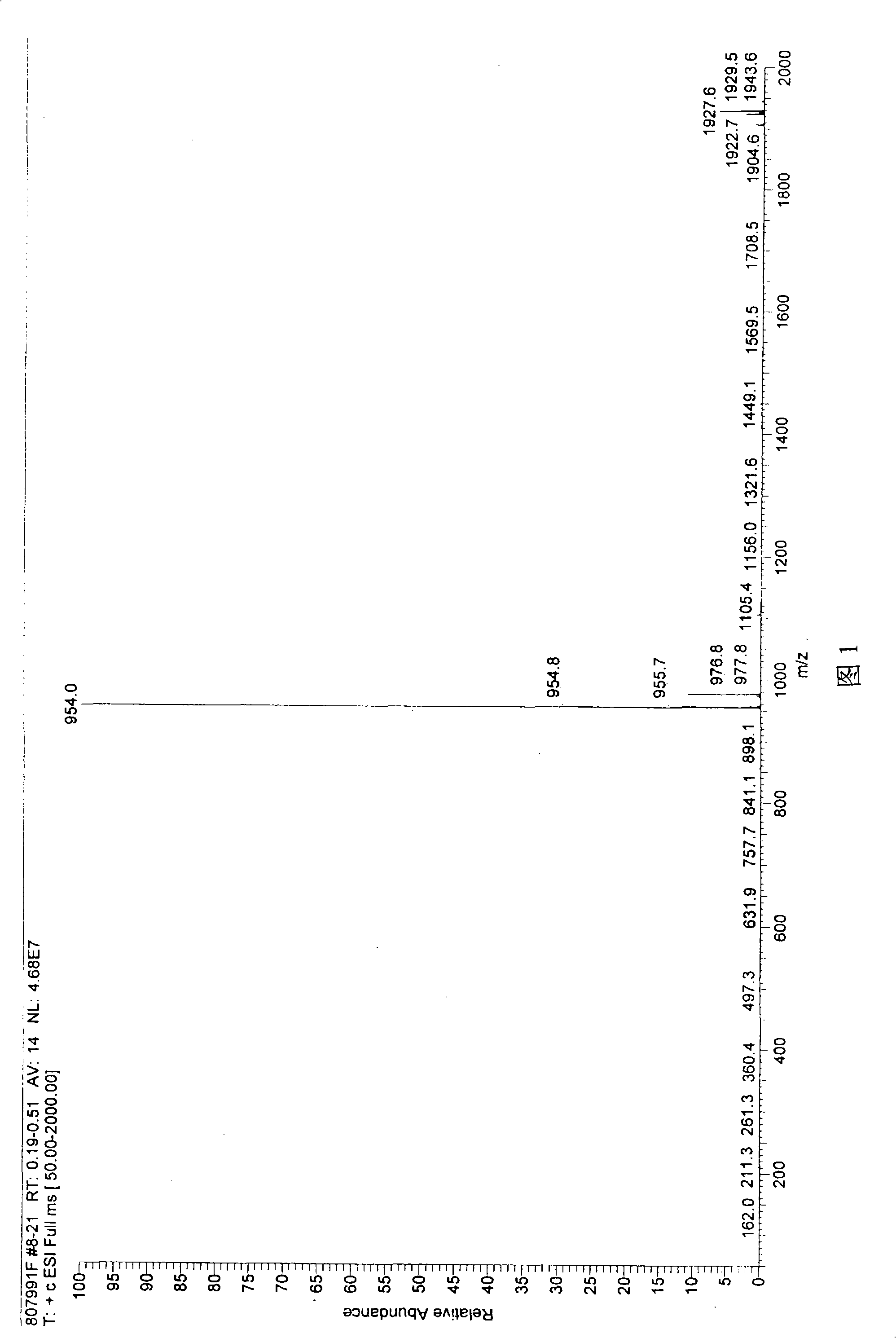
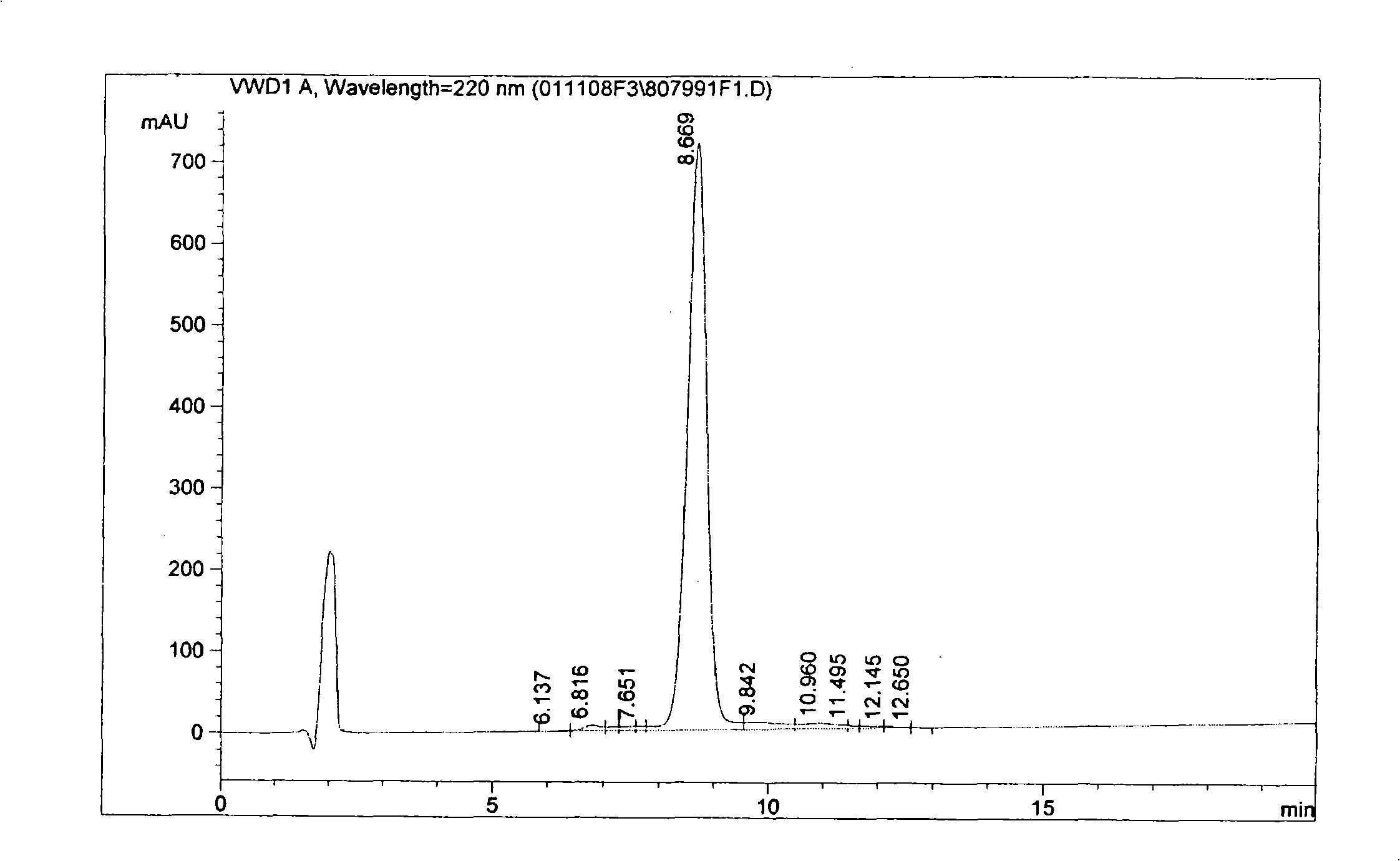
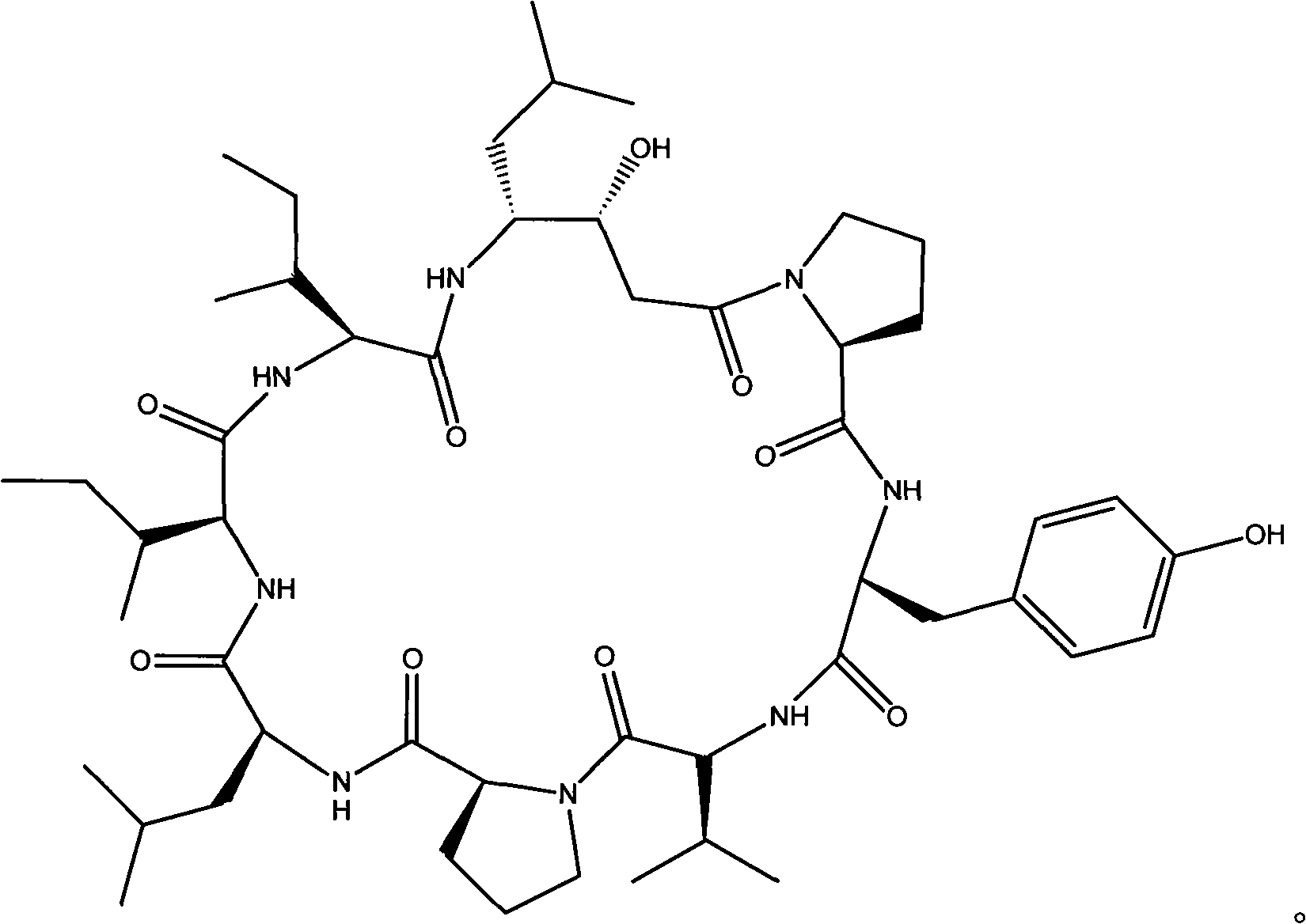
Cyclic peptide with -Ile-Sta-Sta-Pro- residue segment and used as immunity inhibitor and synthetic process thereof
A technology of immunosuppressant and synthesis process, applied in the fields of peptide, organic chemistry, drug combination, etc., can solve the problems of difficult chemical synthesis, unfavorable purification, etc., and achieve the effect of stable physical and chemical properties, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The technical solutions of the present invention will be further specifically described below through examples.
[0028] 1 synthetic part
[0029] 1.1 Instruments and reagents
[0030] Peptide synthesizer (ABI 433, American ABI company), semi-preparative high performance liquid chromatography (WatersDelta Prep 4000, American Waters company), analytical high performance liquid chromatography (Agilent 1100, American Agilent company), freeze dryer ( ChristAlpha, German CHRIST company), ion trap mass spectrometer (LCQ Deca, American Thermo-Finnigan company), ultraviolet spectrophotometer (BeckmanDU7400, American Beckman company), C18 reversed-phase analysis column (Waters XTerra, 3.5um, 4.6×150mm).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com