Process for preparing gemcitabine and associated intermediates
A technology of gemcitabine and isomers, applied in the field of preparation of gemcitabine and related intermediates, can solve the problems of reduced yield and reduced attractiveness, and achieve the effect of being easy to use and promoting total synthesis
Inactive Publication Date: 2008-10-29
CHEMAGIS
View PDF14 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, this method requires additional steps relative to the method given in Scheme 2, which makes it less attractive in terms of industrial feasibility
The production of gemcitabine is inherently problematic, in particular the method requires the preparation and separation of isomers, which tends to result in lower yields on a commercial scale
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
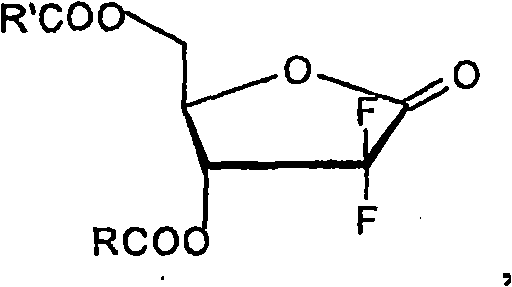
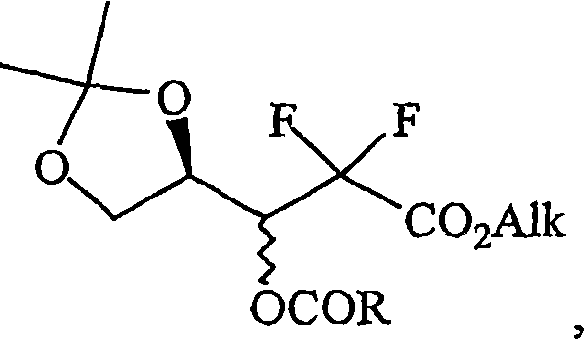
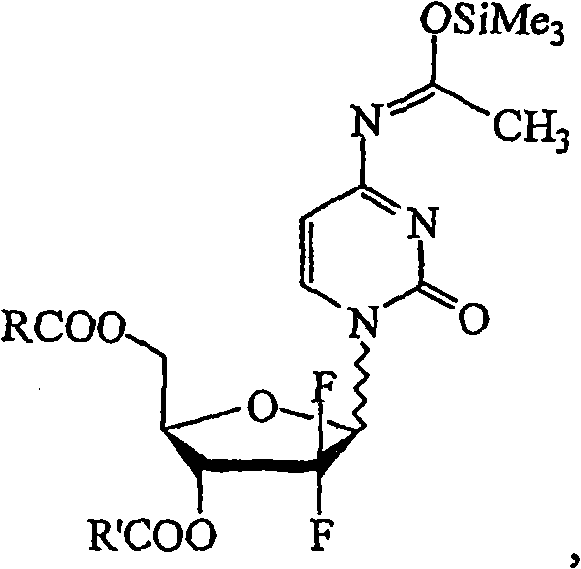
Provided is a process for preparing gemcitabine or a salt thereof, which preferably includes removing at least a substantial portion of the a anomer of a N-1-protected-2'-deoxy-2',2'-difluoro-cytidine-3',5'-diester from an anomeric mixture thereof; removing the 3'-ester, the 5'-ester and the N-protecting group; and optionally forming a salt. The 3'-ester and 5'-ester can be the same or different and at least one of the esters preferably is cinnamoyl, benzoyl, 1-naphthoyl, 1-naphthylmethylcarbonyl, 2-methylbenzylcarbonyl, 4-methylbenzylcarbonyl or 9-fluorenylmethyloxycarbonyl ester. Also provided are novel intermediates, including but not limited to 2-deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-diesters, and methods of producing such intermediates.
Description
Background of the invention Gemcitabine HCl (Gemcitabine HCl), marketed by Eli Lilly under the trade name is a nucleoside analog with antitumor activity, belonging to the large group of chemotherapeutic drugs called antimetabolites. Gemcitabine stops the growth of cancer cells and causes them to die by interfering with the synthesis of nucleic acids, preventing cells from producing DNA and RNA. Gemcitabine is a synthetic glycoside analog of cytosine, which is chemically described as 4-amino-1-(2-deoxy-2,2-difluoro-β-D-ribofuranose)-pyrimidine-2(1H) -keto or 2'-deoxy-2',2'-difluorocytidine (beta isomer). Gemcitabine HCl has the following structure: Gemcitabine HCl 1 Supplied in sterile hydrochloride form in vials for intravenous administration only, containing 200 mg or 1 g of gemcitabine HCl (as the free base) in the form of a sterile lyophilized powder formulated with mannitol ( 200mg or 1g, respectively) and sodium acetate (12.5mg or 62.5mg, respectively). Hydrochlo...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07H19/19
CPCC07H19/067Y02P20/55A61P35/00C07H19/19C07H19/073
Inventor 沈敬山李亚飞J·卡斯比
Owner CHEMAGIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com