Monoazo compounds, preparation method and uses thereof
A monoazo, compound technology, applied in the field of monoazo compounds, can solve the problems of difficulty in accurate color matching, limitation of wide application, intolerant of alkalis, etc., and achieves the effects of low cost, good temperature dependence and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
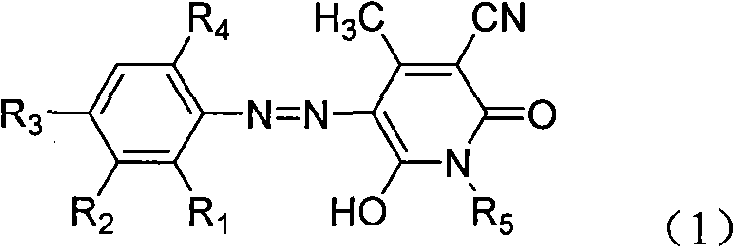
[0040] The aniline compounds shown in formula (2)
[0041]
[0042] In the formula,
[0043] R 1 is hydrogen, C 1-6 Alkyl, halogen, nitro,
[0044] R 2 is hydrogen, C 1-6 alkyl,
[0045] R 3 is hydrogen, C 1-6 Alkyl, halogen, nitro,
[0046] R 4 is hydrogen, C 1-6 Alkyl, halogen, nitro, methoxy,
[0047] That is to have the definition of above-mentioned formula (1), carry out diazotization with nitrosyl sulfuric acid in sulfuric acid medium, the diazonium salt that obtains is coupled with the compound shown in formula (3) in weakly acidic medium,
[0048]
[0049] Wherein R5 is hydrogen, C1-6 alkyl, that is, it has the definition of the above formula (1), and the monoazo compound of the present invention represented by the formula (1) is obtained after suction filtration and separation.
[0050]
[0051] Diazo component formula (2) and coupling component formula (3) can be prepared by well-known methods, or can be obtained commercially.
[0052] The prese...
Embodiment 2
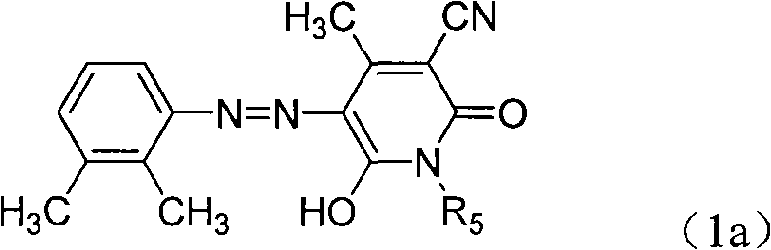
[0057] In reaction bottle, add 200ml water, the industrial hydrochloric acid of 40ml30%, add 2,3-xylidine shown in 18g formula (2a) under stirring,
[0058]
[0059] 120 g of crushed ice was added, 36 g of 30% sodium nitrite solution was added dropwise, and the reaction was incubated for 60 minutes after adding the sodium nitrite to obtain 2,3-xylidine diazonium salt. The heterocyclic compound shown in 24.5g formula (3a)
[0060]
[0061] Dissolve in 8g of soda ash and 600ml of water, add 15g of 60% crystalline sodium acetate, add 200g of crushed ice, add the diazonium salt solution dropwise, after the diazonium salt is added, stir and react for 1 hour, heat up to 65-70°C, keep warm 2 hours. Suction filtration and washing with water gave the compound of the following formula.
[0062]
Embodiment 3
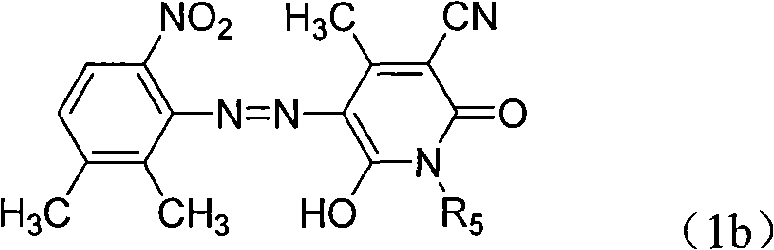
[0064] In reaction bottle, add 200ml water, the industrial hydrochloric acid of 40ml30%, add the 2 shown in 24.5g formula (2b) under stirring, 3-dimethyl-6-nitroaniline,
[0065]
[0066] Add 120 g of crushed ice, add dropwise 36 g of 30% sodium nitrite solution, and keep the reaction for 3 hours after adding the sodium nitrite to obtain 2,3-dimethyl-6-nitroaniline diazonium salt. The heterocyclic compound shown in 24.5g formula (3a)
[0067]
[0068]Dissolve in 8g of soda ash and 600ml of water, add 15g of 60% crystalline sodium acetate, add 200g of crushed ice, add the diazonium salt solution dropwise, after the diazonium salt is added, stir and react for 1 hour, heat up to 65-70°C, keep warm 2 hours. Suction filtration and washing with water gave the compound of the following formula.
[0069]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com