High-stability low-residual suspension solution production device and technique
A low-residue, high-stability technology, applied in the direction of making medicines into special physical or taking forms of devices, drug delivery, pharmaceutical formulations, etc. Dispersion and hydrophilicity, improving stability and reducing residual content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
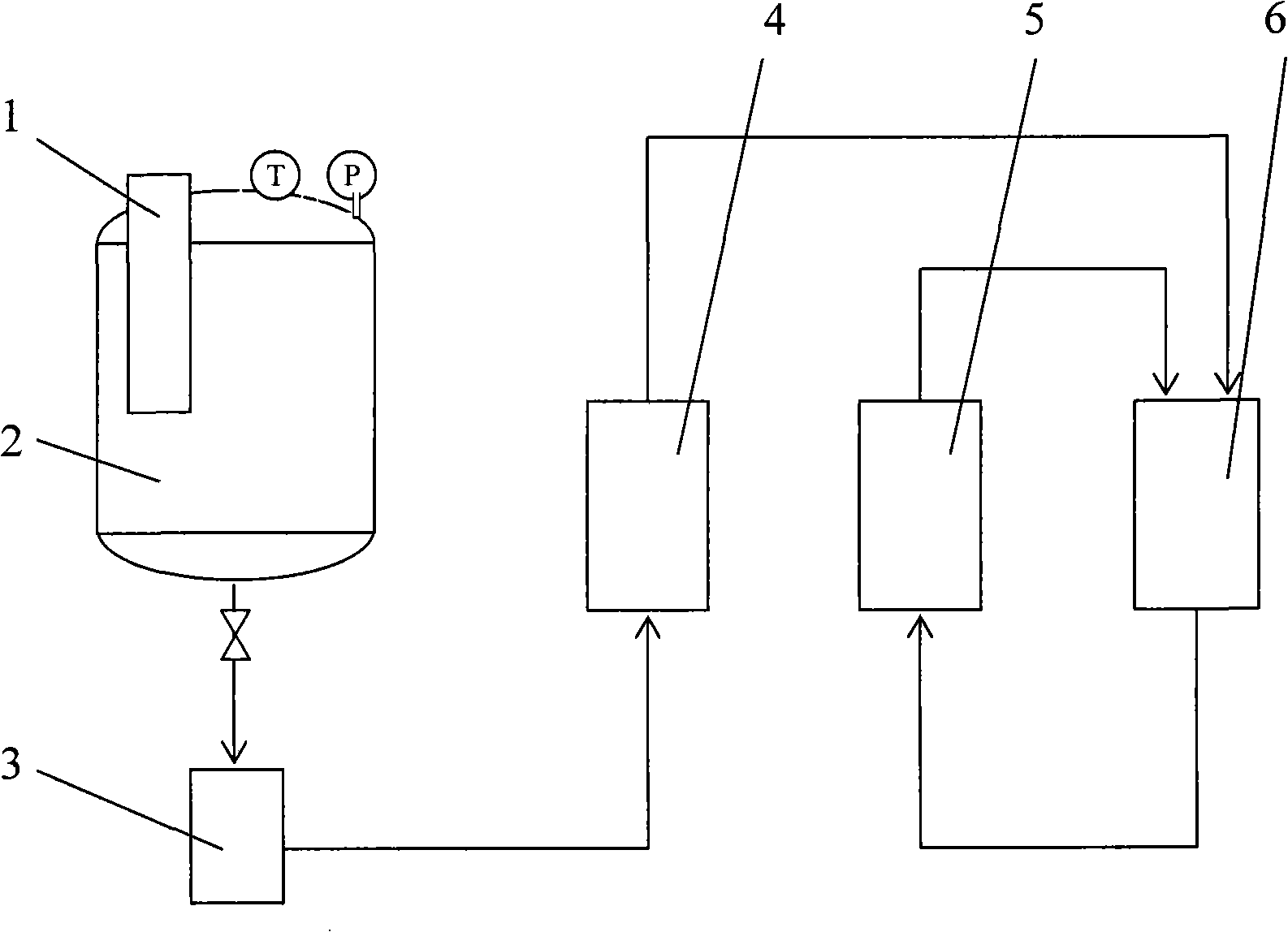
[0011] like figure 1 As shown, the production equipment of the high stability and low residual suspension solution includes a feeder 1, a recrystallization tank 2, a centrifuge 3, a particle extruder 4, a circulating fluidized separator 5 and an electric vacuum oven 6; The feeder 1 is set in the recrystallization tank 2, the recrystallization tank 2 is connected with the centrifuge 3, the centrifuge 3 is connected with the particle extruder 4, the particle extruder 4 is connected with the electric heating vacuum drying box 6, and the electric heating vacuum drying The box 6 is connected with the circulating fluidized separator 5, and the circulating fluidized separator 5 is connected with the electrothermal vacuum drying box 6 again.
[0012] The specific process for making the high-stability and low-residue suspension solution is as follows: place the feeder 1 at the feeding port of the recrystallization tank 2, heat the feeder 1 to 60°C--70°C before feeding, and at the same ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap