Gastric mucosa calibration reagents and preparation method
A calibration solution and gastric mucosa technology, applied in the field of labeling solution and preparation, can solve the problems of not being able to provide long-term, safe and effective labeling, and achieve the effect of long retention time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] A new type of gastric mucosa calibration solution provided by the present invention is prepared from medical Indian ink stock solution and sterile water for injection to a concentration of 1:10 in volume ratio. , the pH value is 8.2, and the carbon content is 0.60%.
[0015] According to the medical requirements of the calibration solution and with reference to domestic and foreign medical literature, various calibration solution raw materials were screened. Finally, we selected the stock solution of Indian ink (Pelikan, Hanover, Germany) imported from Germany, and the quality met the European pharmaceutical standards. The main components of the stock solution are carbon, ethylene glycol, sodium tetraborate decahydrate and ammonia water.
Embodiment 2
[0017] Prepare the calibration solution according to the principles of the lowest concentration, the smallest dose, the longest time and the clearest display. The calibration solution used is medical India ink. After shaking the stock solution of Indian ink, dilute it with sterile water for injection to a concentration of 1:10, 1:100, 1:1000 in a sterile environment, and fill it into a glass vial. Sterilize with moist heat (100°C, 30 minutes) by circulating steam.
[0018] Quality standard: The appearance should be a black suspension, uniform and consistent, without lumps or precipitation. The pH was neutral, and the pH was measured on various dilutions of India ink using a pH meter (825MP, Fisher Scientific, Pittsburgh, PA). The determination of carbon content was carried out by elemental analysis (elemental analysis instrument: ThermoFinnigan, model: Flash EA1112).
[0019] Medical India ink—weighing—drying at 80°C for 48 hours—cooling in a desiccator—weighing—elemental a...
Embodiment 3
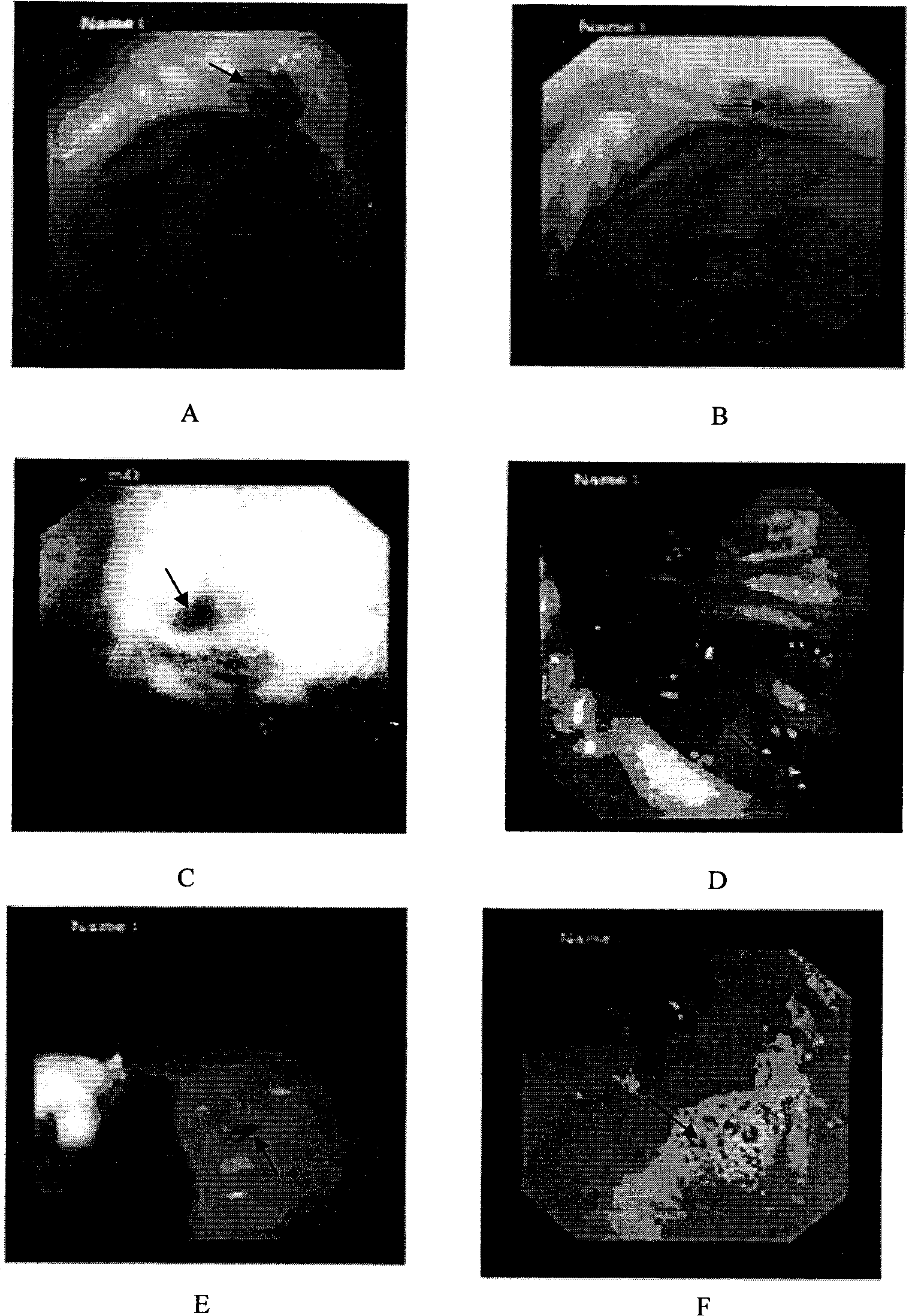
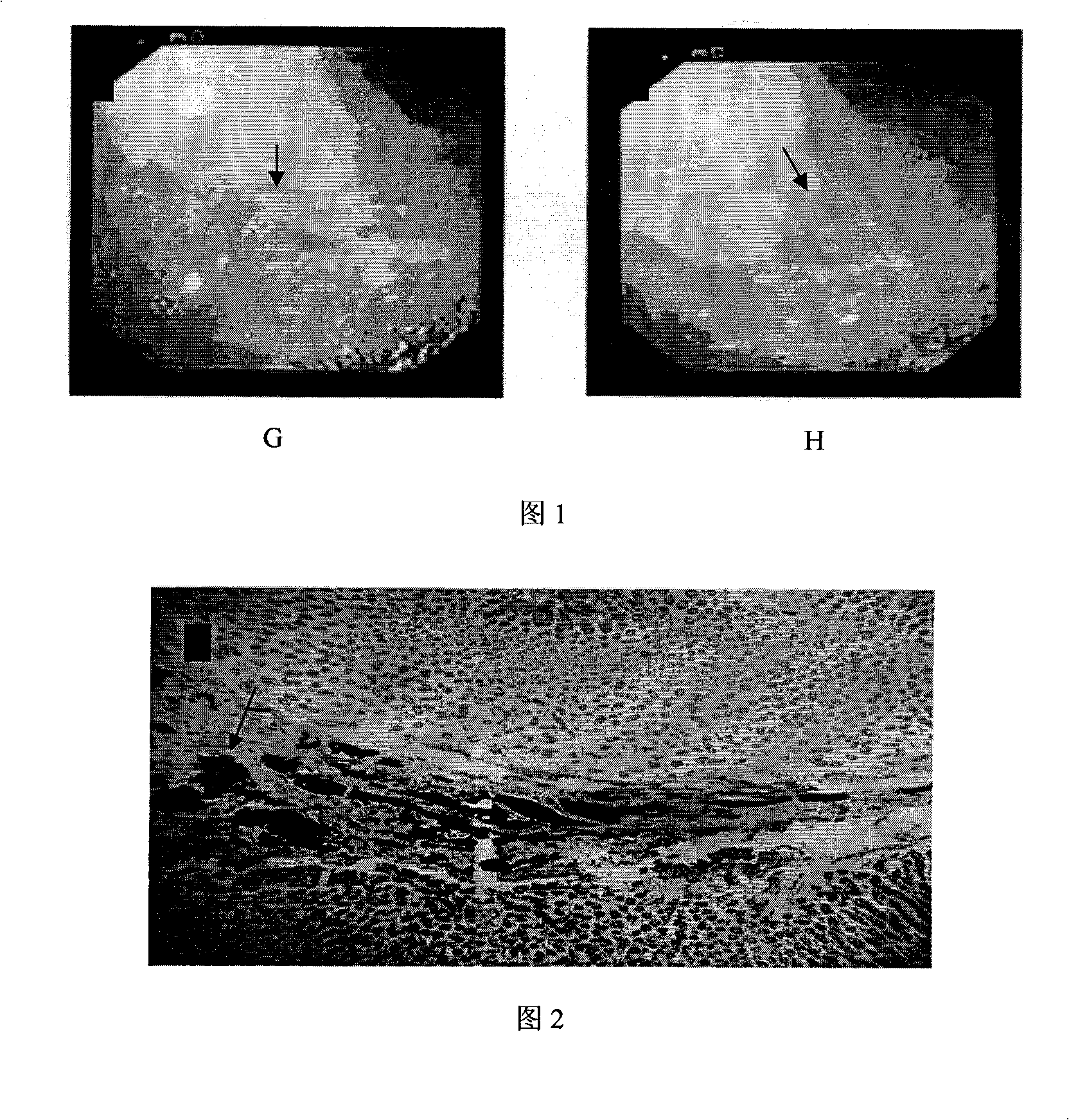
[0046] Embodiment 3 Animal experiments verify the performance, effectiveness and safety of the calibration solution
[0047] To study the clarity and local histological reaction caused by different concentrations and different doses of calibrator. Determine the appropriate concentration and dosage of calibrator, the effectiveness, long-term and safety of the label.
[0048] All animal experiments were completed in the animal laboratory of Run Run Shaw Hospital, which is a national third-level medical animal laboratory.
[0049] 1. New Zealand white rabbit experiment:
[0050] Materials and Methods:
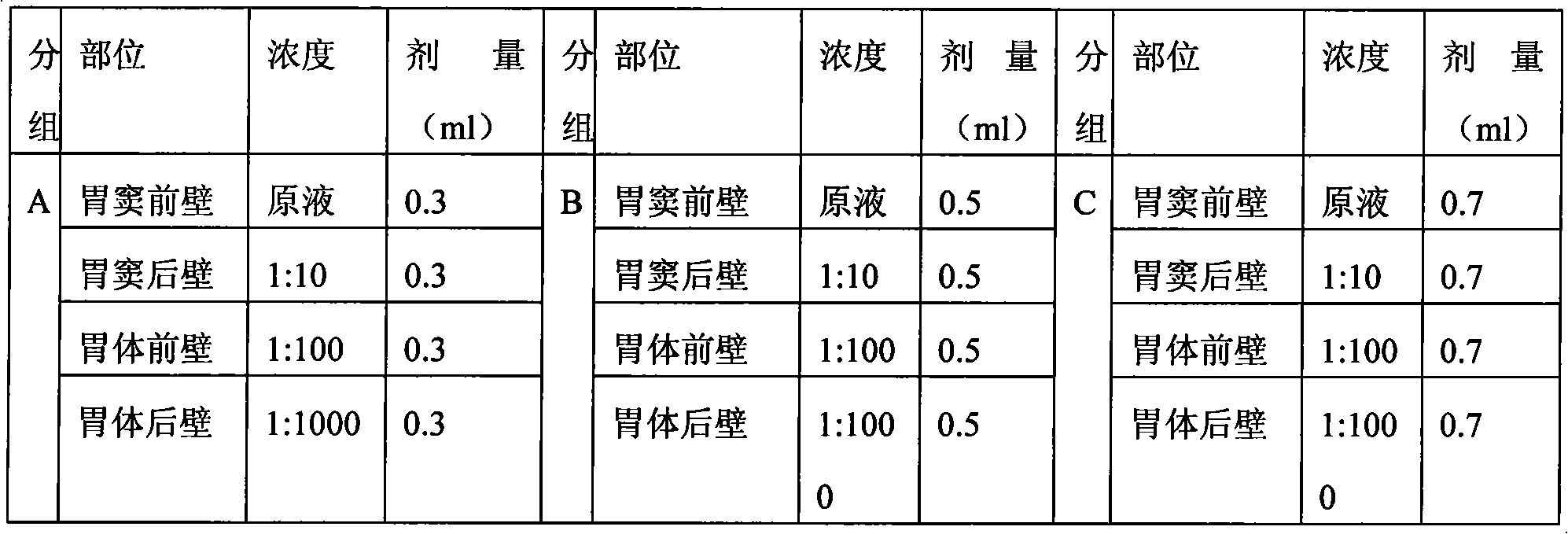
[0051] Calibration by laparotomy. New Zealand white rabbits, adult females, weighing 2-2.5 kg. There are 6 rabbits in total. Two groups were used, the experimental conditions within the group were the same, and the doses between the groups were different. According to the dose of calibration solution injected at each point, it was divided into three groups: A, B, and C. Each...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com