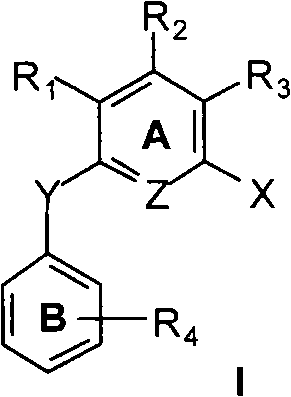
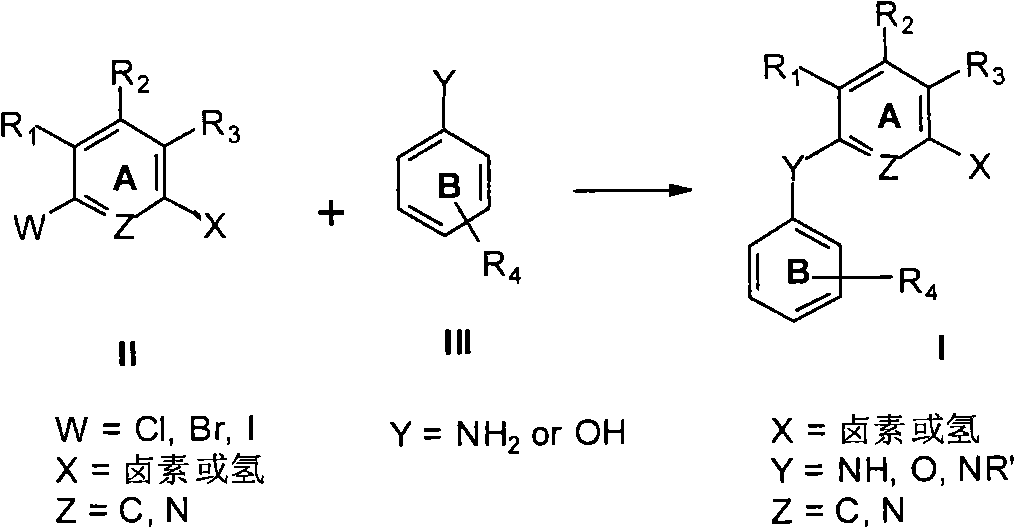
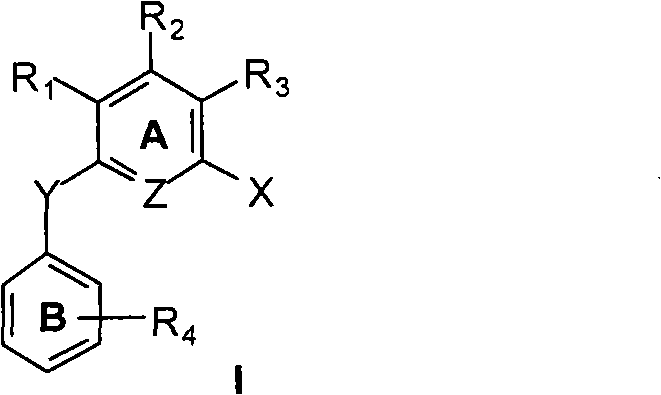
N-substituted arene aniline / polysubstituted diaryl ether compound, preparation and anti-tumor use thereof
A technology of diaryl ether and aromatic hydrocarbon aniline, which is applied in the field of preparation of anti-tumor drugs, and can solve the problems of unsatisfactory treatment and endangering human life and health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Example 1: N-(m-hydroxyphenyl)-5-chloro-2,4-dinitroaniline (A1)
[0091] Dissolve 1,5-dichloro-2,4-dinitrobenzene (237mg 1.0mmol) and m-aminophenol (230mg, 2.11mmol) in DMF (0.5mL) and place it directly in an oil bath at 120°C, stirring for 2 Minutes, the reaction solution turned red and cooled to room temperature, adding ice water and dilute HCl to adjust the pH to 3. The solid was filtered out, and the crude product was separated with a silica gel plate, developing solvent: petroleum ether / ethyl acetate, to obtain 208 mg of the title compound as a red solid, with a yield of 67%. 1 H NMR (DMSO) δppm 6.77 (1H, s, ArH-2'), 6.78 (1H, d, J=8.4Hz, ArH-4'), 6.81 (1H, t, J=8.4Hz, ArH-5' ), 7.04 (1H, s, ArH-6), 7.31 (1H, d, J=8.4Hz, ArH-6'), 8.90 (1H, s, ArH-3), 9.83 (1H, s, OH), 10.04 (1H, s, NH).
Embodiment 2
[0092] Example 2: 5-chloro-N-(4'-cyanophenyl)-2,4-dinitroaniline (A2)
[0093] 2,4-Dichloro-1,5-dinitrobenzene (1.2 g, 5 mmol) and p-aniline (0.59 g, 5 mmol) were dissolved in N,N-dimethylformamide (DMF, 10 mL). Potassium tert-butoxide (1.4 g, 12.5 mmol) was added in batches in an ice-water bath, followed by stirring at room temperature for 45 minutes. The reaction solution was poured into ice water, and the pH was adjusted to neutral with dilute HCl. The precipitated solid was filtered off, washed with water, and dried. The crude product was separated on a silica gel column (ethyl acetate / petroleum ether) to obtain the title compound (1.44 g, 90%) as a pale yellow solid. 1 H NMR (CDCl 3 )δppm, 7.41 (1H, s, ArH-6), 7.57 (2H, d, J=8.8Hz, ArH-3', 5'), 7.91 (2H, d, J=8.8Hz, ArH-2', 6'), 8.90 (1H, s, ArH-3), 10.07 (1H, s, NH).
Embodiment 3
[0094] Example 3: 5-chloro-2,4-dinitro-N-(4'-methoxyphenyl)-aniline (A3)
[0095] Dissolve 2,4-dichloro-1,5-dinitrobenzene (0.5 g, 2.11 mmol) and p-methoxyaniline (0.26 g, 2.11 mmol) in DMSO (5 mL), add K 2 CO 3(0.58g, 4.22mmol) and a catalytic amount of metallic Cu were stirred at 115°C for 2 hours under nitrogen protection. Pour into ice water, filter out the solid, wash with water several times, dry the crude product and separate it with a silica gel column (eluted with petroleum ether / ethyl acetate) to obtain the title compound as a reddish-brown solid, 0.68g, yield 67%. 1 H NMR (CDCl 3 )δppm 3.88 (3H, s, OCH 3 ), 7.02 (1H, s, ArH-6), 7.03 (2H, d, J=8.96Hz, ArH-3', 5'), 7.21 (2H, d, J=8.96Hz, ArH-2', 6 '), 9.07 (1H, s, ArH-3), 9.73 (1H, s, NH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com