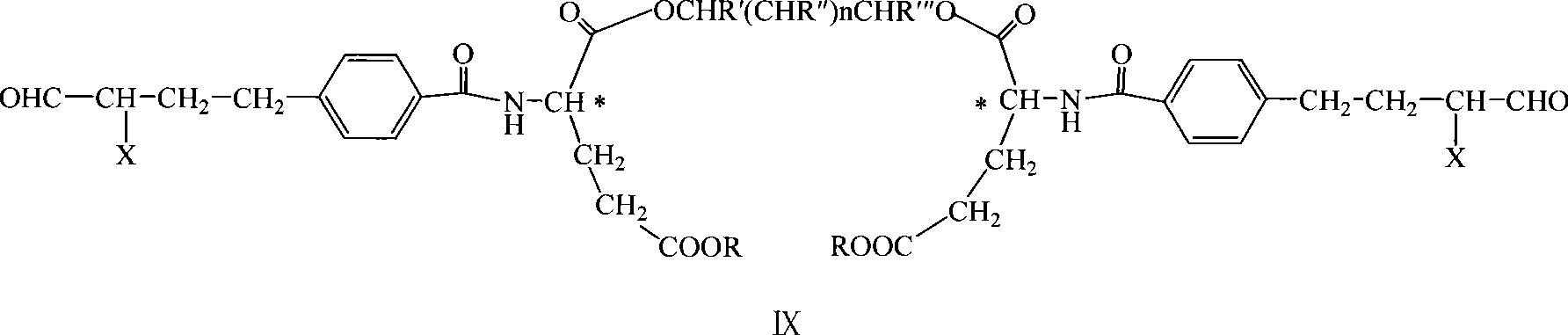
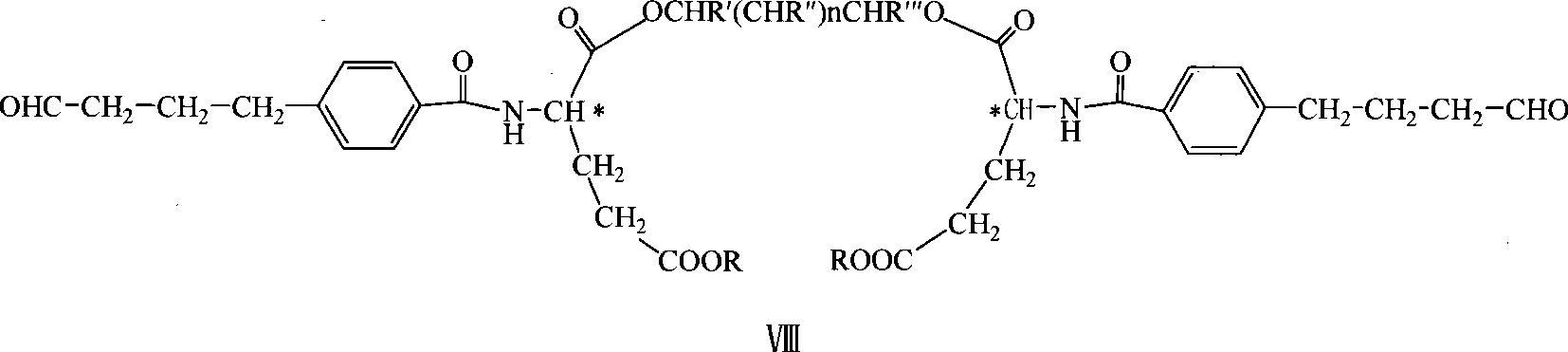
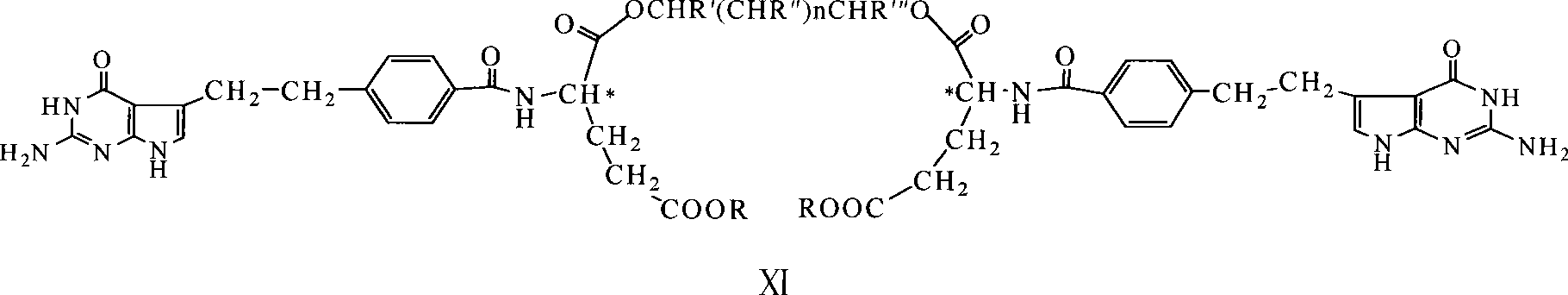Diglutamate derivates and application thereof in preparation of pemetrexed
A technology of diglutamate and glutamate, which is applied in the field of medicine and chemical industry, can solve the problems of strong volatility of bromine, long reaction steps, and many reaction by-products, and achieve easy industrial production, mild reaction conditions, and high product quality. The effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of N-(p-iodobenzoyl)-L-glutamic acid-γ-methyl ester (IV):
[0067]
[0068] method one:
[0069] Dissolve 100g (0.403mol) of p-iodobenzoic acid in 500ml of DMF, stir at room temperature, add 61g (0.604mol) of N-methylmorpholine, 2,4-dimethoxy-6-chloro-1,3 , 5-s-triazine 105.7g (0.604mol), stirred at room temperature for 1h, TLC analysis of raw materials disappeared, 1000ml ethyl acetate and 1000ml water extraction separation, sodium sulfate drying, sodium sulfate was removed by suction filtration, ethyl acetate was removed under reduced pressure , proceed directly to the next step.
[0070] Dissolve 90g of L-glutamic acid in 600ml of methanol, add 60g of concentrated sulfuric acid, dissolve completely, then add 3ml of concentrated sulfuric acid and stir overnight at room temperature, TLC analysis shows that the raw material disappears, use 1N sodium hydroxide to adjust pH = 7, remove the product by suction filtration salt, proceed directly to the next r...
Embodiment 2
[0077] Preparation of N-(p-iodobenzoyl)-L-glutamic acid-γ-methyl ester-α-(4-bromobutyl ester) (VI′):
[0078]
[0079] 20g (51.2mmol) of N-(p-iodobenzoyl)-L-glutamic acid-γ-methyl ester was dissolved in 200ml of DMF, and stirred at room temperature to completely dissolve; add 1,4-dibromobutane 110g (509mmol), 22 g (205 mmol) of sodium carbonate (205 mmol) and 20 g of tetrabutylammonium bromide were vigorously stirred at room temperature for 4 h, and the raw materials disappeared according to TLC analysis. 400ml of ethyl acetate and 400ml of water were extracted and separated, and the aqueous layer was extracted with ethyl acetate; the organic phases were combined and washed with water; dried over anhydrous sodium sulfate, filtered to remove anhydrous sodium sulfate, evaporated under reduced pressure to remove the solvent, and separated by column chromatography , to obtain 26.2 g of white solid, yield 97.2%.
[0080] 1 HNMR (DMSO-6, 600M) δ: 8.835(s, 1H), 7.885(d, 2H), 7.6...
Embodiment 3
[0082] Preparation of two [N-(p-iodobenzoyl)-L-glutamic acid-γ-methyl ester]-1,4-butanediester (VI):
[0083]
[0084] 26.5g (50.4mmol) N-(p-iodobenzoyl)-L-glutamic acid-γ-methyl ester-(4-bromobutyl ester), N-(p-iodobenzoyl)-L-glutamic acid -23.7g (60.5mmol) of γ-methyl ester, 25.7g (242mmol) of sodium carbonate, and 16.2g (50.4mmol) of tetrabutylammonium bromide were dissolved in 100ml of DMF, heated at 100°C for 3h, and the raw materials disappeared by TLC analysis. Extract with 300ml ethyl acetate and 300ml water, extract the aqueous layer with ethyl acetate, combine the organic phases, wash with water, dry over anhydrous sodium sulfate, remove anhydrous sodium sulfate, remove the solvent under reduced pressure, recrystallize ethyl acetate to obtain a white solid 33.6g product, yield 80.1%.
[0085] 1 HNMR (DMSO-6, 600M) δ: 8.827 (d, 2H), 7.860 (d, 4H), 7.643 (d, 4H), 4.410 (m, 2H), 4.062 (m, 4H), 3.578 (s, 6H) ), 2.453(t, 4H), 2.074(m, 4H), 1.068(m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com