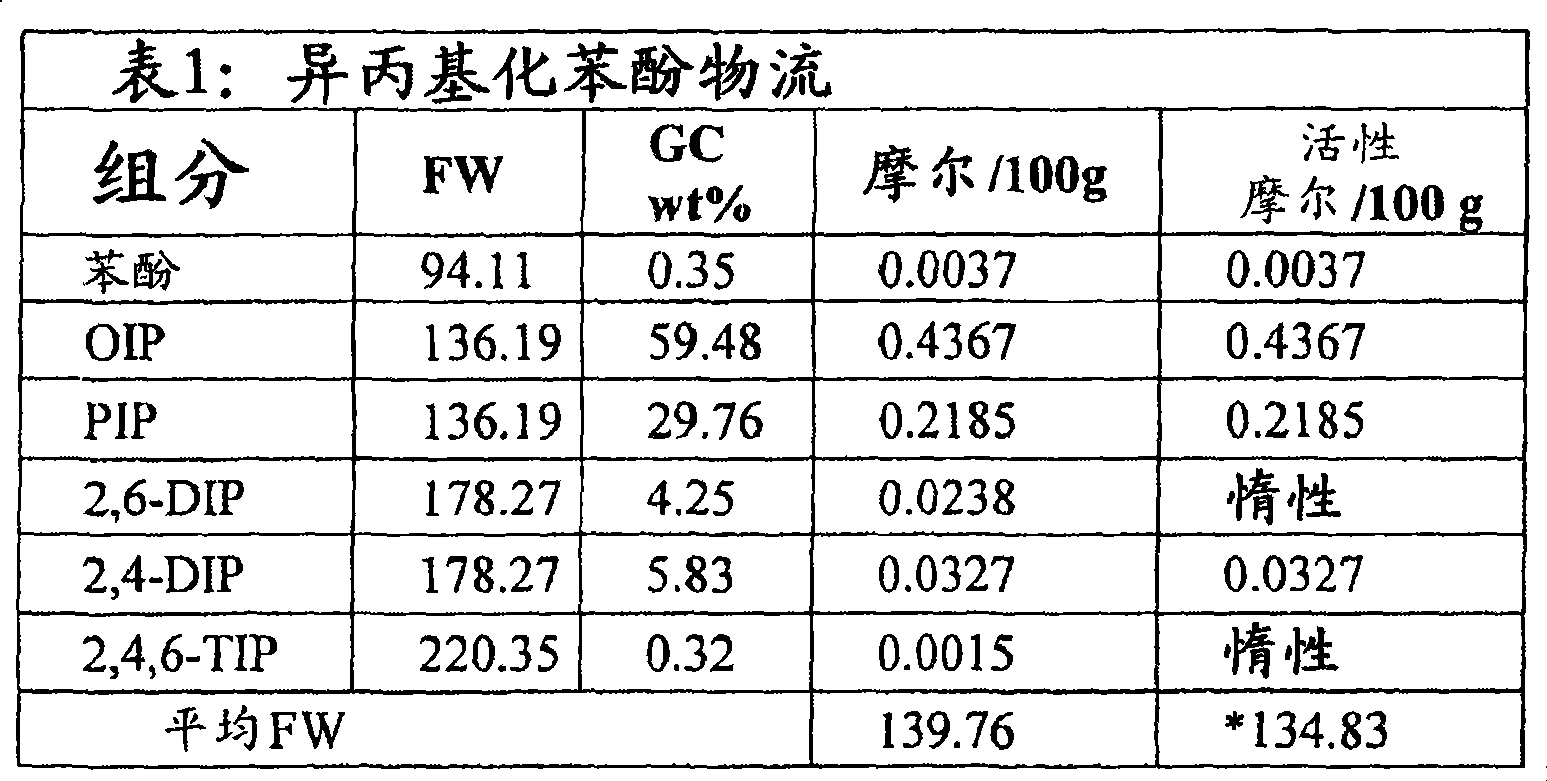
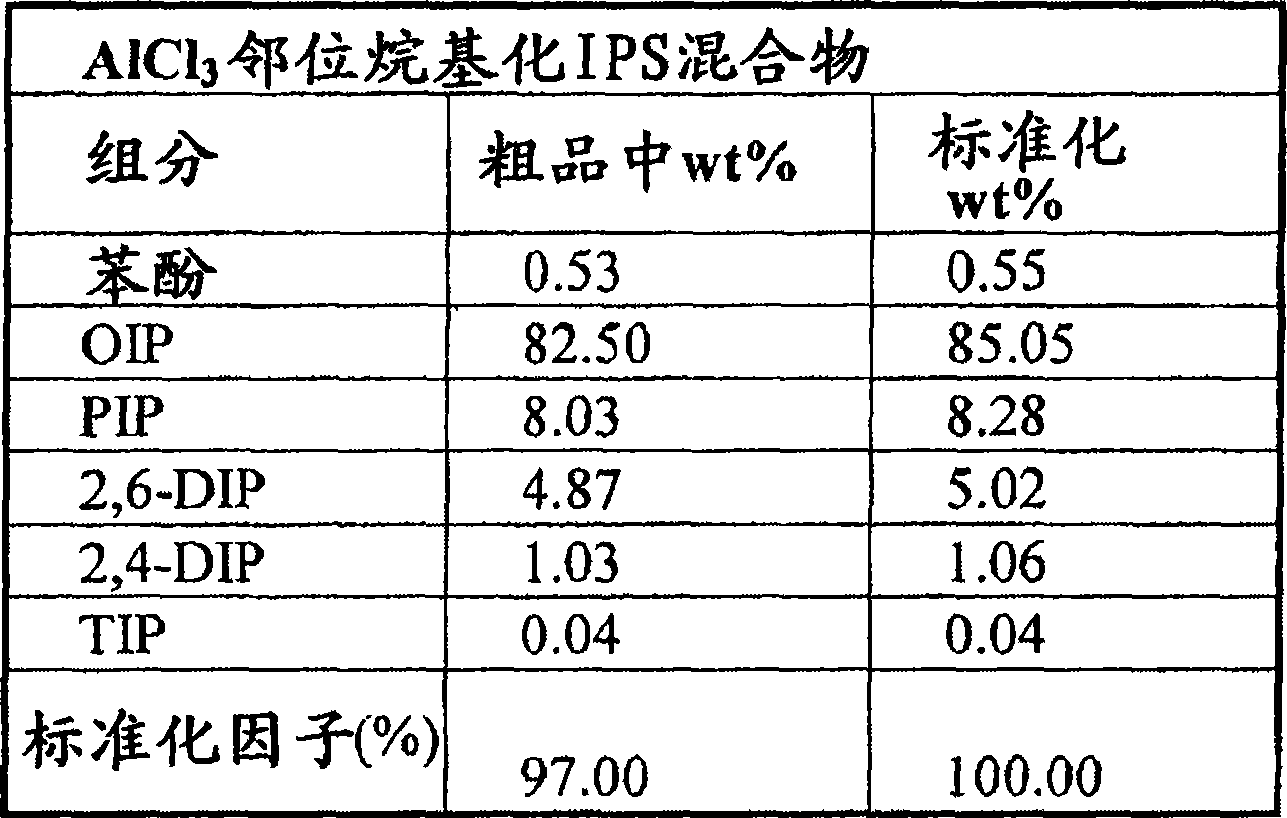
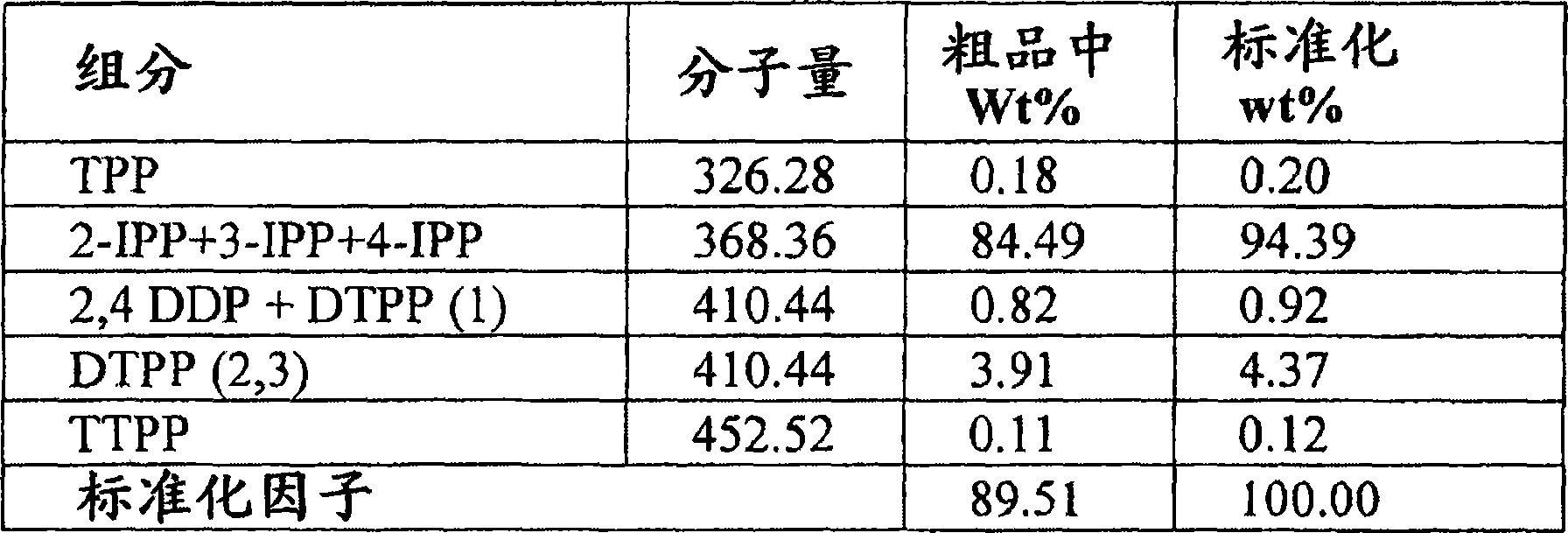
Low triphenylphosphate, high phosphorous content isopropyl phenyl phosphates with high ortho alkylation
A technology of trialkyl phenyl phosphate and alkyl phenyl diphenyl phosphate, which can be applied in the fields of compounds of Group 5/15 elements of the periodic table, organic chemistry, chemical instruments and methods, etc., and can solve problems such as reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1 (comparative)
[0085]The reaction flask was purged with nitrogen. 15.3g (0.1 mole) phosphorus oxychloride ("POCl 3 ") This was followed by 13.6 g (0.1 mole) of o-isopropylphenol ("OIP"). The mixture was heated to about 110°C for 10 hours with stirring. 1 H-NMR analyzed the contents of the flask and found that more than 50 mol% of OIP was unreacted. Also with the help of 31 P-NMR analyzed the contents of the flask, and found that the molar ratio of 2-cumylphenyl dichlorophosphate to bis(2-cumylphenyl)chlorophosphate to tris(2-cumylphenyl)phosphate It is 40.8:22.6:5.0.
Embodiment 2
[0086] Example 2 (comparative)
[0087] The reaction flask was purged with nitrogen. Then 15.3 g (0.1 mole) of phosphorus oxychloride ("POCl 3 ") followed by 13.6 g (0.1 mol) of o-isopropylphenol ("OIP"). The mixture was heated to 195°C for 5 hours with stirring. The contents of the flask were analyzed by means of proton NMR, and it was detected by this analysis The presence of unreacted OIP in the flask indicates that the reaction is incomplete. Then the contents of the flask are heated to 250°C for 3 hours with stirring until no OIP is detected. 31 P-NMR analyzed the components in the flask, and found that the molar ratio of 2-cumylphenyl dichlorophosphate to bis(2-cumylphenyl)chlorophosphate to tris(2-cumylphenyl)phosphate was 56.2:28.7:2.8.
Embodiment 3
[0088] Example 3 (Example 1-Comparison from US 4,139,487)
[0089] Phenol (65.2 parts) and a mixture of meta- and p-isopropylphenol (47.9 parts) were mixed with phosphorus oxychloride (51 parts; that is to say 5% excess of phenolic reactants). The reaction was catalyzed by the addition of crushed anhydrous magnesium chloride (0.5 parts). The reaction mixture was rapidly heated to 130°C and then slowly heated to 230°C over a period of about 2 hours, after which no further hydrogen chloride escaped significantly. The reaction is checked by titration test on the crude product, and then the crude product is vacuum distilled to produce recovered phenol fraction, a small amount of middle distillate and main ester fraction (88% of crude product) boiling at 205℃~225℃ under 1mm mercury column. .
[0090] Analysis shows that the recovered phenol fraction is basically the same as the phenol feed mixture, indicating that there has been no obvious component separation due to preferential este...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Kinematic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com