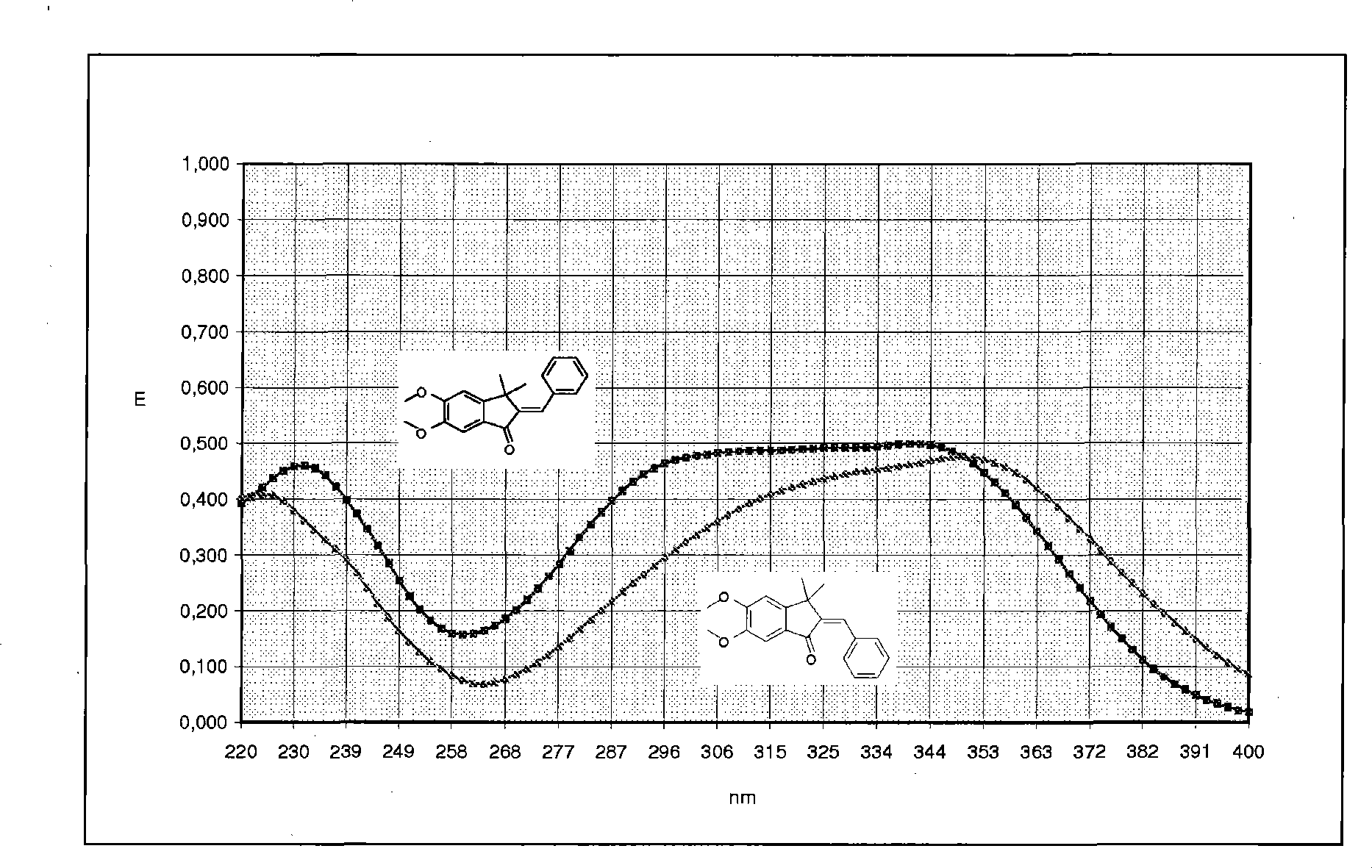
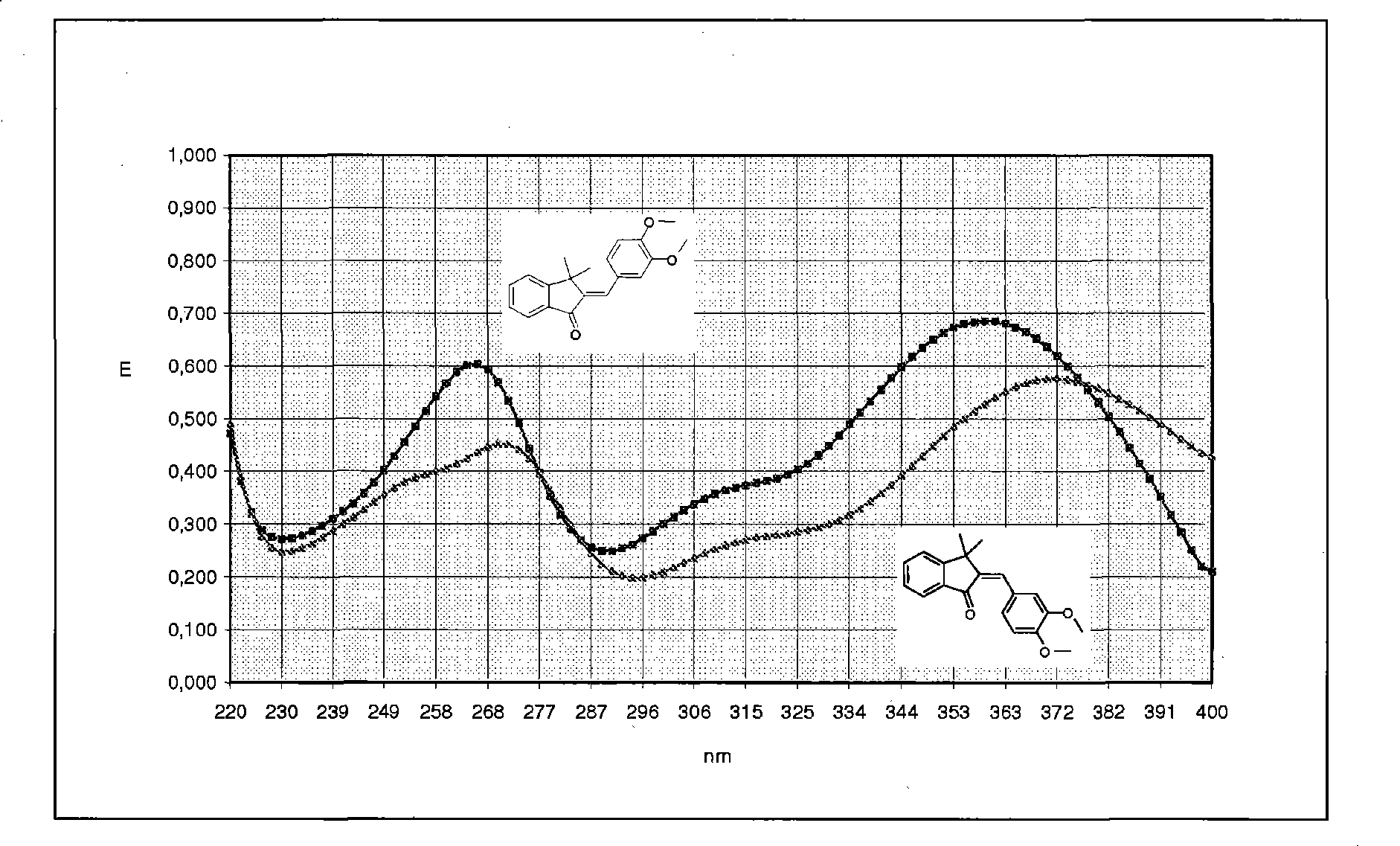
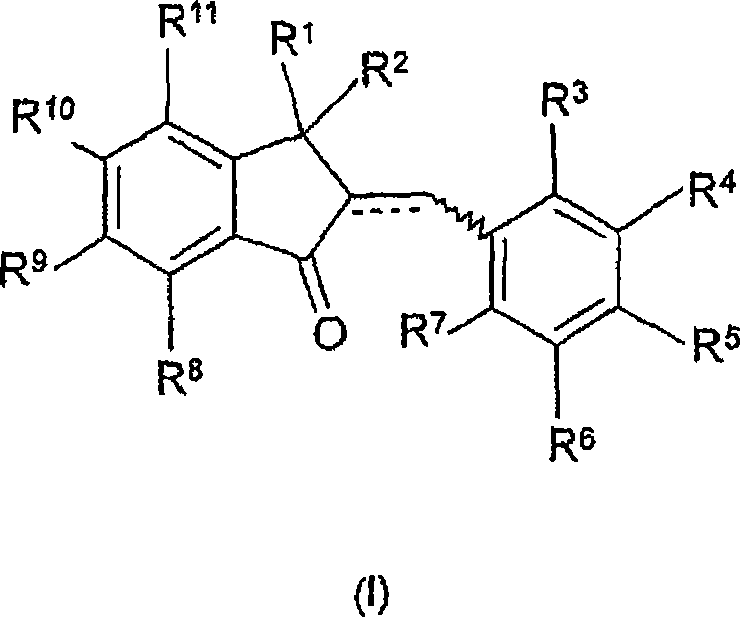
Ah receptor antagonists
A preparation and filter technology, applied in the field of aromatic hydrocarbon receptors (Ah receptors, can solve problems such as enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 6
[0363] Embodiment 6 (decolorization)
[0364] B16V mouse melanoma cells were divided into 5×10 3 Cells / well were seeded into 96-well microtiter plates. at 37°C and 5% CO 2 After 24 hours of incubation in RPMI matrix enhanced with 10% fetal bovine serum, different concentrations of test substances, 0.3 mM tyrosine and 10 nM α-MSH (α-melanocyte stimulating hormone) were added and incubation was continued for 96 hours. Standards were incubated with kojic acid at concentrations of 0.01 mM, 0.1 mM and 1 mM with tyrosine and α-MSH. Only tyrosine and α-MSH were added to the control. After incubation, sodium lauryl sulfate and sodium hydroxide solutions (final concentrations of 1 mM and 1 M, respectively) were added to the medium, and the absorbance (A) was measured at 400 nm after 3 hours.
[0365] Staining inhibition in the presence of test compound or kojic acid was calculated using the following equation:
[0366] Coloring inhibition (%)=100-[(A 试验化合物 / A 对照 )×100]
[036...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com