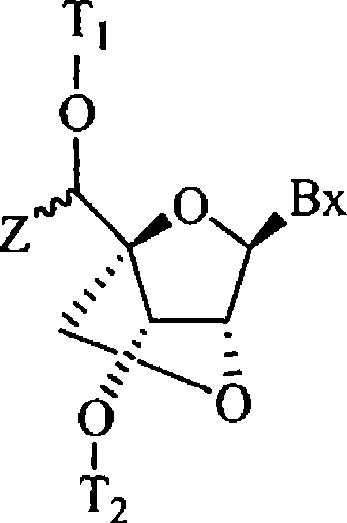
5'-modified bicyclic nucleic acid analogs
A kind of technology of bicyclic nucleosides and compounds, applied in the field of oligomers or compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] Representative U.S. patents that teach the preparation of representative motifs include, but are not limited to: 5,013,830, 5,149,797, 5,220,007, 5,256,775, 5,366,878, 5,403,711, 5,491,133, 5,565,350, 5,623,065, 5,652,355, 5,652,3706, some of which; This application is commonly owned, each of which is incorporated herein by reference in its entirety. Motif filed on June 2, 2005 and published on December 22, 2005 as WO 2005 / 121371 International Application PCT / US2005 / 019219 and filed on June 2, 2005 and published on December 22, 2005 It is also disclosed in PCT / US2005 / 019220 as WO 2005 / 121372, each of which is incorporated herein by reference in its entirety.
[0054] The terms "stable compound" and "stable structure" refer to a compound that is sufficiently robust to be isolated to a useful degree of purity from a reaction mixture and formulated as an effective therapeutic agent. Only stable compounds are concerned here.
[0055] Selected substituent groups within the c...
Embodiment 1
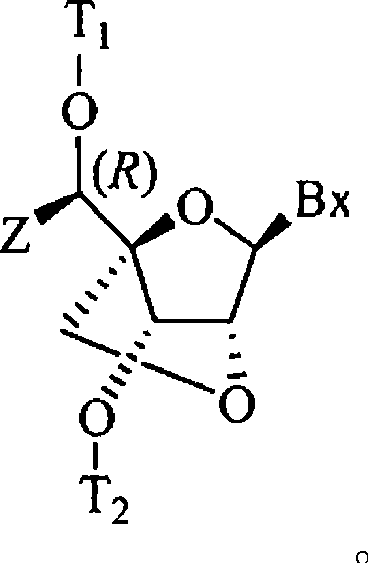
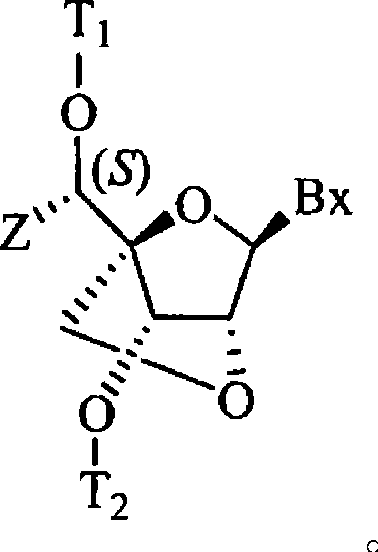
[0132] (1R, 3R, 4R, 7S)-7-[2-cyanoethoxy(diisopropylamino)phosphineoxy]-1-[1-(S)-(4,4'-dimethoxy Preparation of Trityl)oxy-ethyl]-3-(uracil-1-yl)-2,5-dioxa-bicyclo[2.2.1]heptane (19a)
[0133]
[0134] Route 1(a) TBSCl, Et 3 N,DMAP,CH 2 Cl 2 , room temperature, 16h(b) oxalyl chloride, DMSO, Et 3 N, CH 2 Cl 2 , -78°C to room temperature (c) MeMgBr, CeCl 3 , THF, -78°C (d) isobutyryl chloride, Et 3 N,DMAP,CH 2 Cl 2 , room temperature, 16h(e) 70% HF / pyridine, room temperature, 16h(f) methanesulfonyl chloride, Et 3 N,DMAP,CH 2 Cl 2 (g) AcOH, Ac 2 O, concentrated H 2 SO 4 (h) Uracil, BSA, TMSOTf, CH 3 CN, reflux, 2h (i) NaOH, water, dioxane (j) isobutyric anhydride, DMAP, pyridine (k) Pd / C, H 2 Balloon (1) TBSCl, Imidazole, DMF(m)K 2 CO 3 , MeOH(n)DMTCl, 2,6-lutidine, pyridine, 45℃(o)Et 3 N.3HF, Et 3 N,THF(p)(iPr 2 N) 2 POCH 2 CH 2 CN, tetrazole, NMI, DMF.
[0135] A) Preparation of Compound 4
[0136] A solution of tert-butyldimethylsilyl chloride (6.2...
Embodiment 2
[0170] (1R, 3R, 4R, 7S)-7-[2-cyanoethoxy(diisopropylamino)phosphineoxy]-1-[1-(R)-(4,4'-dimethoxy Preparation of Trityl)oxy-ethyl]-3-(uracil-1-yl)-2,5-dioxa-bicyclo[2.2.1]heptane, compound 19b (Scheme 1)
[0171] A) Preparation of compound 7b
[0172] To compound 6b (1.90 g, 4.4 mmol), triethylamine (0.88 mL, 6.3 mmol) and 4-dimethyl-aminopyridine (53 mg, 0.4 mmol) in ice (0 °C) CH 2 Cl 2 To the solution (5 mL) was added isobutyryl chloride (0.55 mL, 5.2 mmol). After stirring at room temperature for 16 hours, the reactant was poured into EtOAc, and washed successively with 5% aqueous HCl, saturated NaHCO 3 , brine, and dry (Na 2 SO 4 ) and concentrated in vacuo to give compound 7b, which was used in the next step without any purification.
[0173] B) Preparation of Compound 8b
[0174] 70% HF / pyridine (2.0 mL) was added to a solution of crude compound 7b in THF (30 mL) in a polypropylene tube. After stirring at room temperature for 16 hours, triethylamine (2.0 mL) was add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com